Preparation method of drug-carrying hydroxylapatite microspheres and bone cement composite porous microspheres
A technology of hydroxyapatite and nano-hydroxyapatite, which is applied in the fields of phosphorus compounds, chemical instruments and methods, and medical science, and can solve problems such as the influence of in-situ drug loading, difficult drug loading, and unstable drug release. , to achieve the effects of easy regulation of microsphere size, control of drug release rate, and adjustable drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
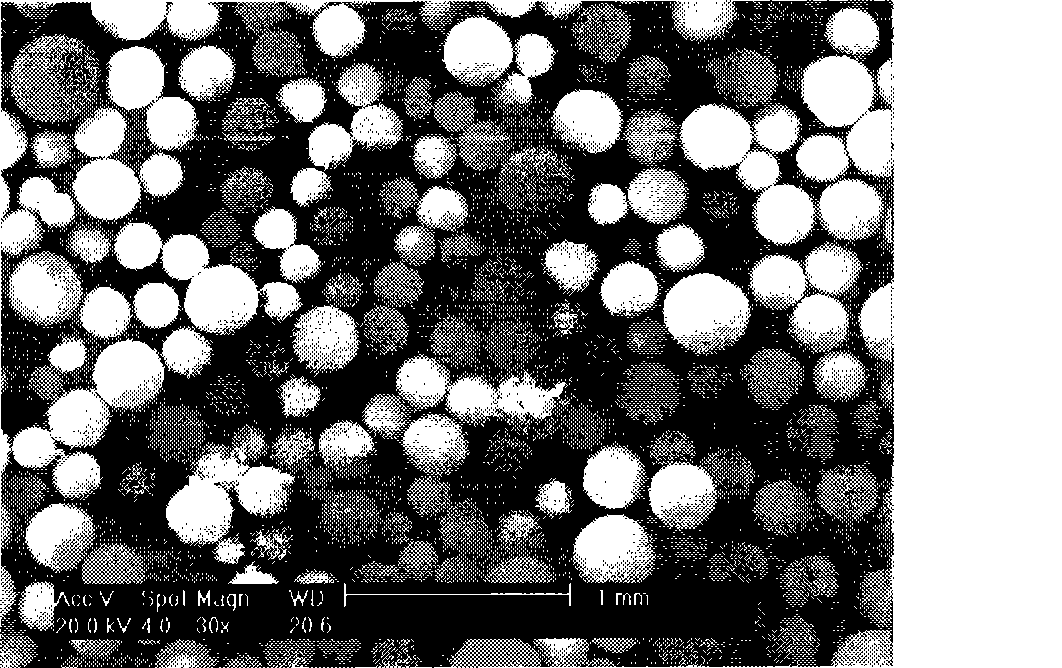
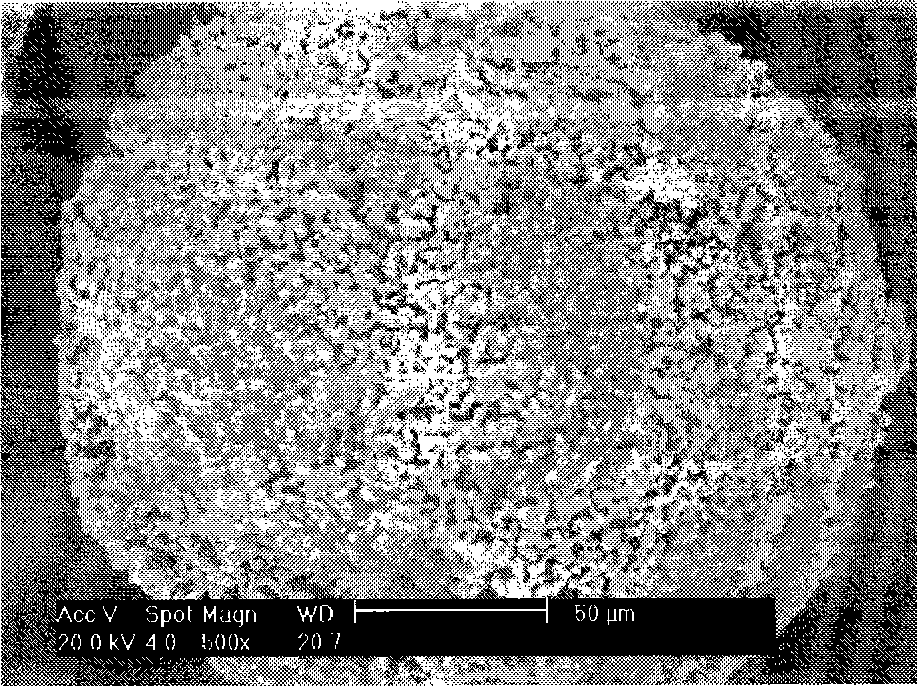
Image
Examples
Embodiment 1
[0018] Step 1: Synthesis of nano-hydroxyapatite
[0019] Accurately weigh 23.66gCa(NO 3 ) 2 ·4H 2 O and 4.26g P 2 O 5 , The corresponding Ca(NO 3 ) 2 ·4H 2 O and P 2 O 5 Dissolved into 300ml and 125ml absolute ethanol, respectively, prepared into the molar concentration of 0.3mol / L and 0.24mol / L Ca(NO 3 ) 2 And P 2 O 5 Solution. Using 100ml absolute ethanol as the base liquid, put P 2 O 5 And Ca(NO 3 ) 2 The solution was dripped into the base liquid at the same time, stirred continuously to obtain a transparent solution, and stood for 24 hours to obtain a milky white gel-like system. It was then dried in an oven at 70°C to obtain a white powder. Then put the white powder obtained in a calcination furnace and heat to 300°C to obtain hydroxyapatite with a low degree of crystallinity. Calcined at 600°C, hydroxyapatite with complete crystals can be obtained, and the calcined powder has good dispersibility. .
[0020] Step two:
[0021] Accurately weigh 171.1 grams of calcium hydrogen p...
Embodiment 2
[0027] Step one and step two are the same as in Example 1.
[0028] Step 3: Preparation of hydroxyapatite porous microspheres
[0029] Dissolve 4.5g of gelatin in 30ml of deionized water at a temperature of 40°C and stir for 1 hour to prepare a gelatin solution with a concentration of 0.15g / ml; accurately weigh out 9g of nano-hydroxyapatite prepared in step 1 of Example 1. Stone (HAp) powder was added to the above gelatin solution to prepare a gelatin / HAp mixed suspension with a concentration of 0.3 g / ml. The gelatin / HAp mixed suspension was added to soybean oil at a stirring speed of 700 rpm, and the oil temperature was kept at 15°C. After stirring for 1 hour, let the microspheres settle on the bottom of the oil layer by standing for 24 hours; pour the upper layer of vegetable oil, wash the prepared microspheres with acetone and ethanol respectively, and dry them naturally in the air to obtain gelatin / HA composite microspheres; place the composite microspheres on the bottom of th...
Embodiment 3
[0033] Step one and step two are the same as in Example 1.
[0034] Step 3: Preparation of hydroxyapatite porous microspheres
[0035] Dissolve 8g of gelatin in 40ml of deionized water at a temperature of 40°C and stir for 1 hour to prepare a gelatin solution with a concentration of 0.2g / ml; accurately weigh 16g of the nano-hydroxyapatite prepared in step 1 of Example 1 ( HAp) powder is added to the above gelatin solution to prepare a gelatin / HAp mixed suspension with a concentration of 0.4 g / ml. The gelatin / HAp mixed suspension was added to soybean oil at a stirring speed of 700 rpm, and the oil temperature was kept at 15°C. After stirring for 1 hour, let the microspheres settle for 36 hours at the bottom of the oil layer; pour the upper layer of soybean oil, wash the prepared microspheres with acetone and ethanol, and dry them naturally in the air to obtain gelatin / HA composite microspheres; place the composite microspheres on the bottom of the oil layer. Sintering is carried ou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com