Para nitro toluylene visible light photosencitizer, synthesis and uses thereof
A technology of nitrostilbene and visible light, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of no absorption and low initiation efficiency, and achieve suitable yield, convenient source of raw materials, and The effect of readily available sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
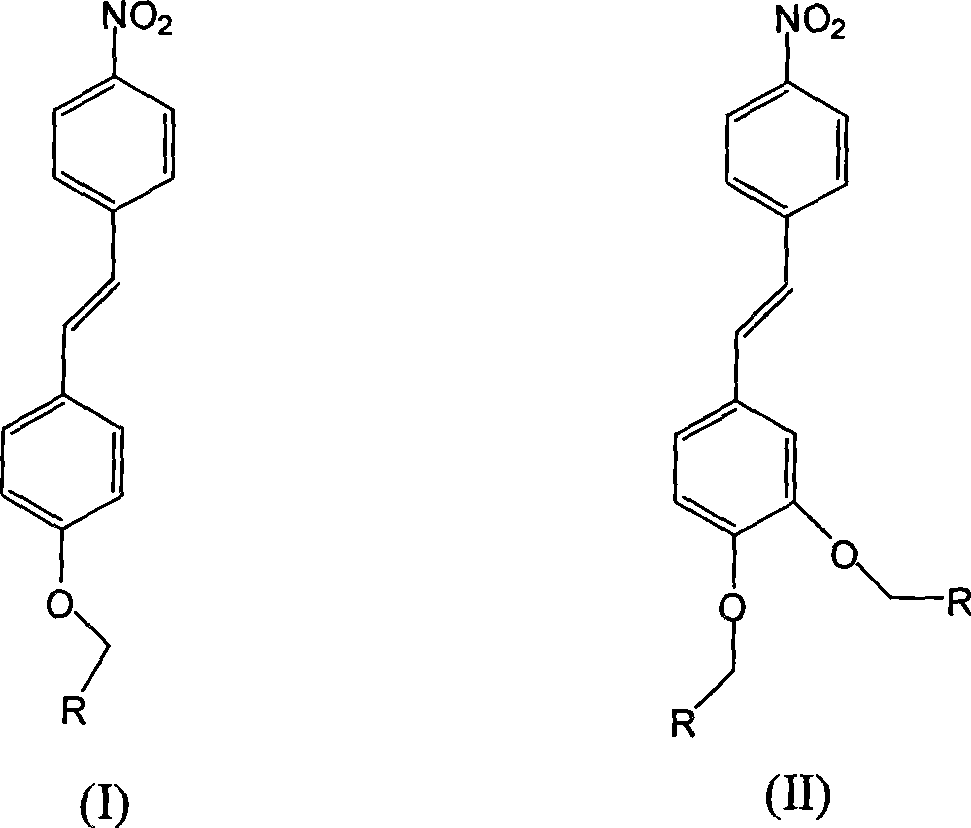
[0034] Synthesis of (p-nitrostilbene)-(toluene)-ether
[0035] The synthesis proceeds in two steps:
[0036] (1) Synthesis of 4-hydroxyl-p-nitrostilbene
[0037] Add compound p-nitrophenylacetic acid (5.0g), 4-hydroxybenzaldehyde (5.1g) and hexahydropyridine (3.5g or 4.1ml) in a three-necked flask (p-nitrophenylacetic acid: 4-hydroxybenzaldehyde: six Hydropyridine (1:1.5:1.5) mixed, heated to 100 ℃ reflux reaction for 3-4 hours, until no bubbles are generated. Then the temperature was raised to 130° C., and the reaction was continued for 2 hours. The reactant was recrystallized twice from hot absolute ethanol to obtain the target compound (4.8 g) with a yield of 72%.
[0038] (2) Synthesis of (p-nitrostilbene)-(toluene)-ether
[0039] Add compound 4-hydroxyl-p-nitrostilbene (2.0g), benzyl bromide (2.1g or 1.5ml), potassium carbonate (2.3g) into a three-necked flask (4-hydroxyl-p-nitrostilbene: benzyl Bromine: Potassium carbonate (1:1.5:2) was mixed, then dissolved in 150m...
Embodiment 2
[0041] Synthesis of (p-nitrostilbene)-(xylene)-ether
[0042] The synthesis proceeds in two steps
[0043] (1) Synthesis of 3,4-dihydroxy-p-nitrostilbene
[0044] Add compound p-nitrophenylacetic acid (5.0g), 3,4-dihydroxybenzaldehyde (5.7g) and hexahydropyridine (4.1ml) in a three-necked flask (p-nitrophenylacetic acid: 3,4-dihydroxybenzene Formaldehyde: Hexahydropyridine (1:1.5:1.5) mixed, heated to 100°C and refluxed for 3-4 hours, until no bubbles were generated. Then the temperature was raised to 130° C., and the reaction was continued for 2 hours. The reactant was recrystallized twice from hot absolute ethanol to obtain the target compound (5.0 g) with a yield of 70%.
[0045] (2) Synthesis of (p-nitrostilbene)-(xylene)-ether
[0046]Add compound 3,4-dihydroxy-p-nitrodiphenylethylene (2.0g), benzyl bromide (4.0g or 2.8ml), potassium carbonate (4.5g) (4-hydroxyl-p-nitrodiphenyl Ethylene: benzyl bromide: potassium carbonate (1:3:4) were mixed, then dissolved in 150ml ...
Embodiment 3
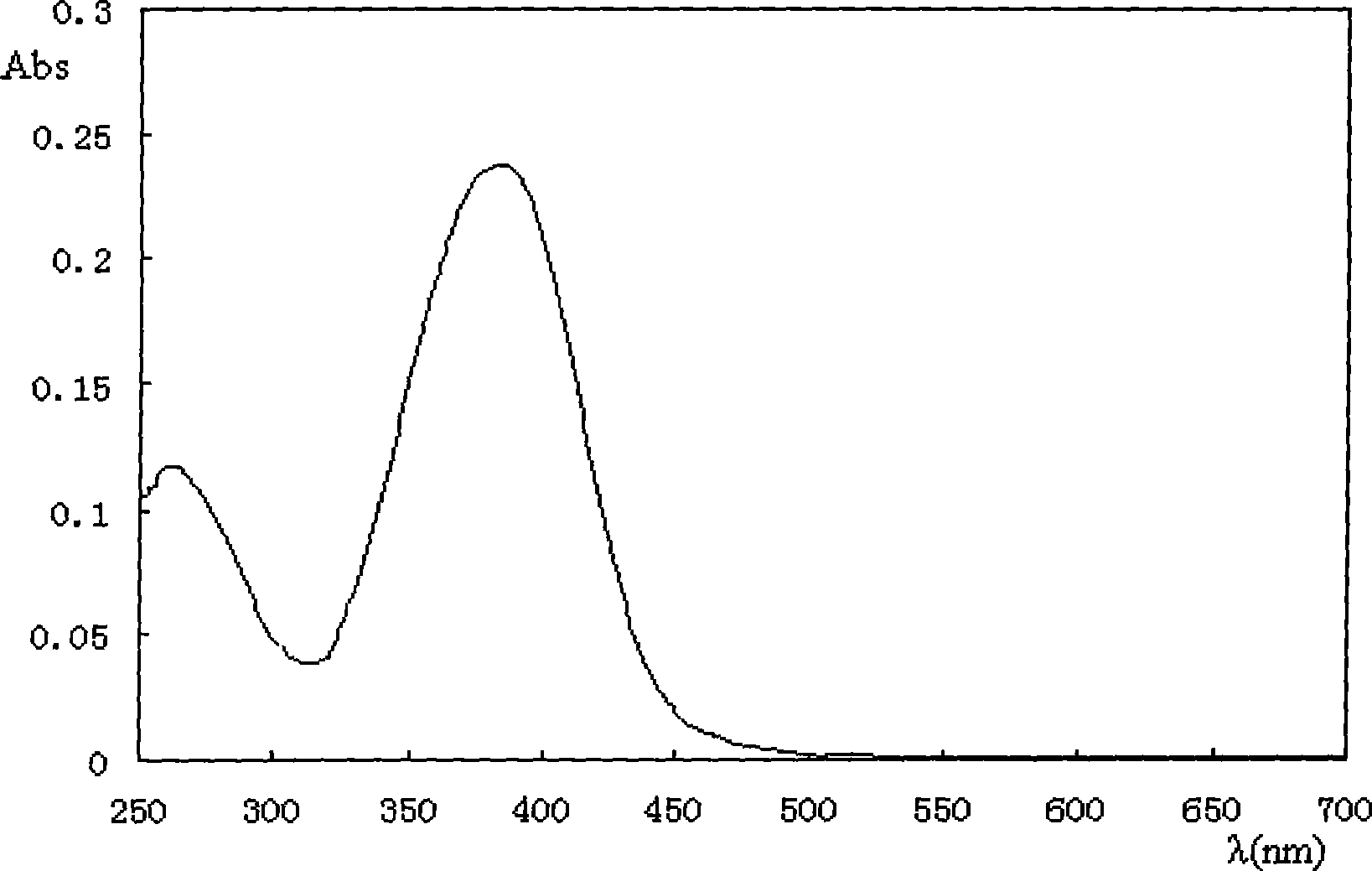
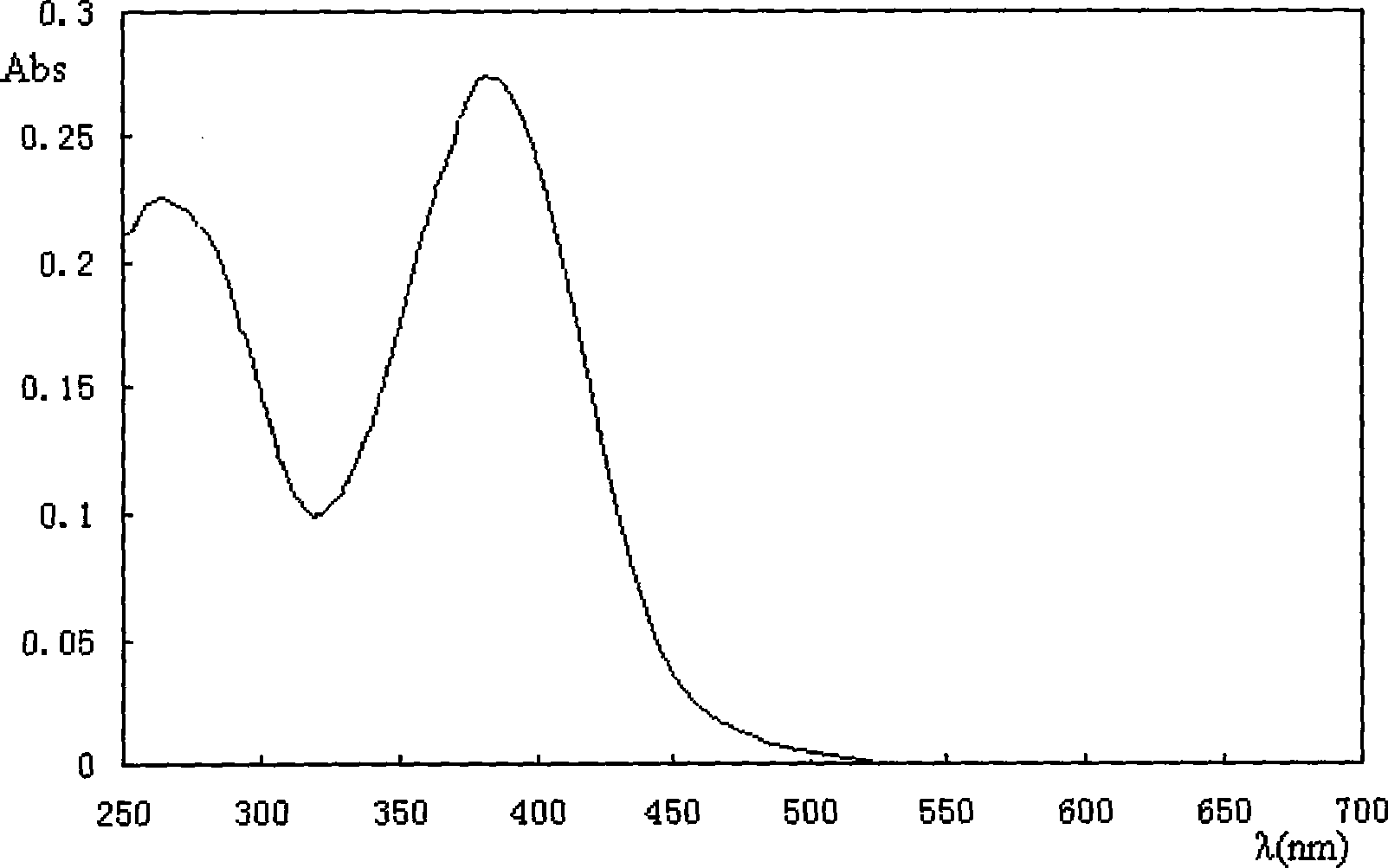
[0048] Will 1×10 -5 The (p-nitrostilbene)-(toluene)-ether of mol / L is dissolved in methylene chloride, and its ultraviolet-visible absorption spectrum is measured, and its maximum absorption is at 381nm, such as figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com