Method for preparing anatase titanic oxide sol
A technology of titanium dioxide and anatase phase, applied in the fields of titanium dioxide, chemical instruments and methods, titanium oxide/hydroxide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] After heating 100mL of 1mol / L titanium sulfate solution to 60°C, keep it warm at 60°C, add dropwise 400mL of 1mol / L sodium hydroxide solution, neutralize until the pH value is approximately equal to 7, keep it at 60°C for 4 hours, and obtain a white precipitate. Wash repeatedly with distilled water to obtain a gel-like solid. Take 80 grams of the wet precipitate (12 grams converted into titanium dioxide) and disperse it in 100 ml of 1 mol / L hydrochloric acid. Disperse the gel at 60 ° C for 2 hours to obtain a light blue transparent Sol, the concentration of titanium dioxide is 0.92mol / L.
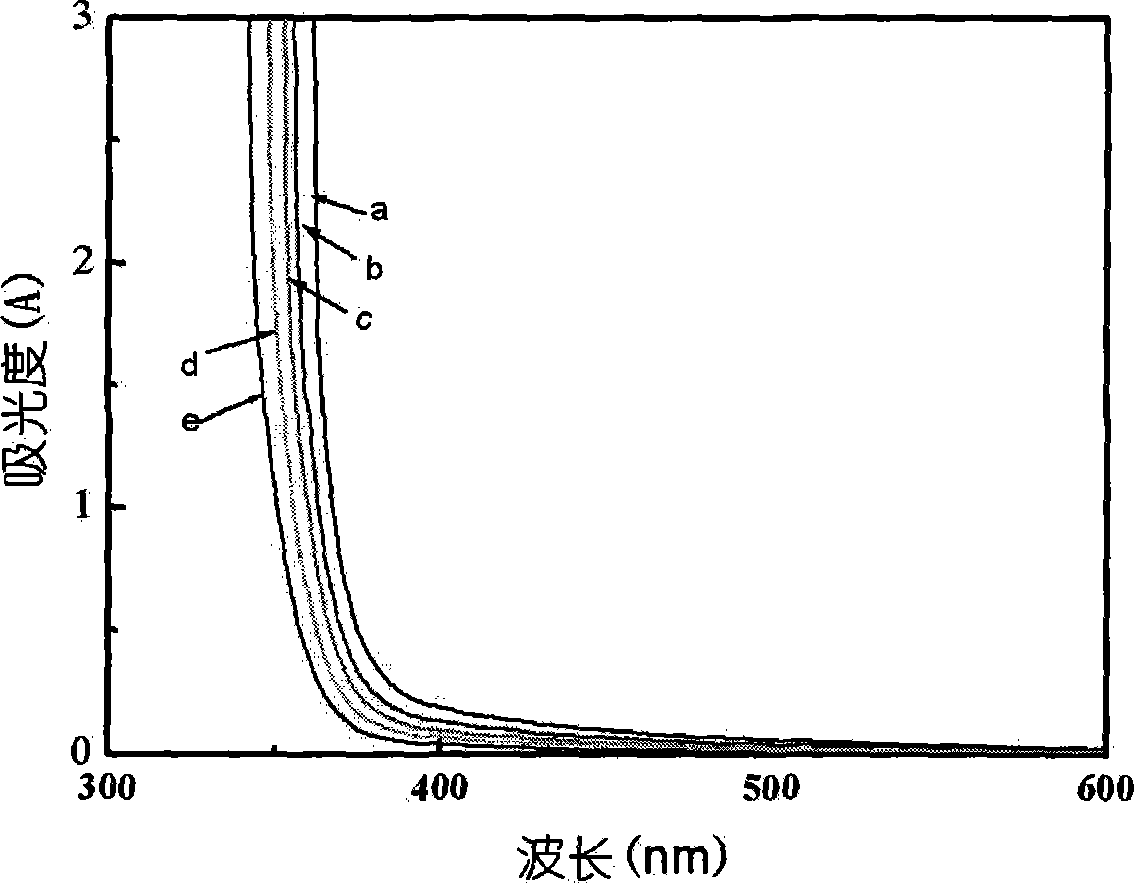
[0041] The concentration of hydrogen ions before and after degumming was measured, and it was found that the hydrogen ions were not consumed, indicating that the degumming had nothing to do with the dissolution of orthotitanic acid. After diluting different times with deionized water, its ultraviolet-visible absorption spectrum is as follows: figure 1 As shown, there is no absorption...
Embodiment 2
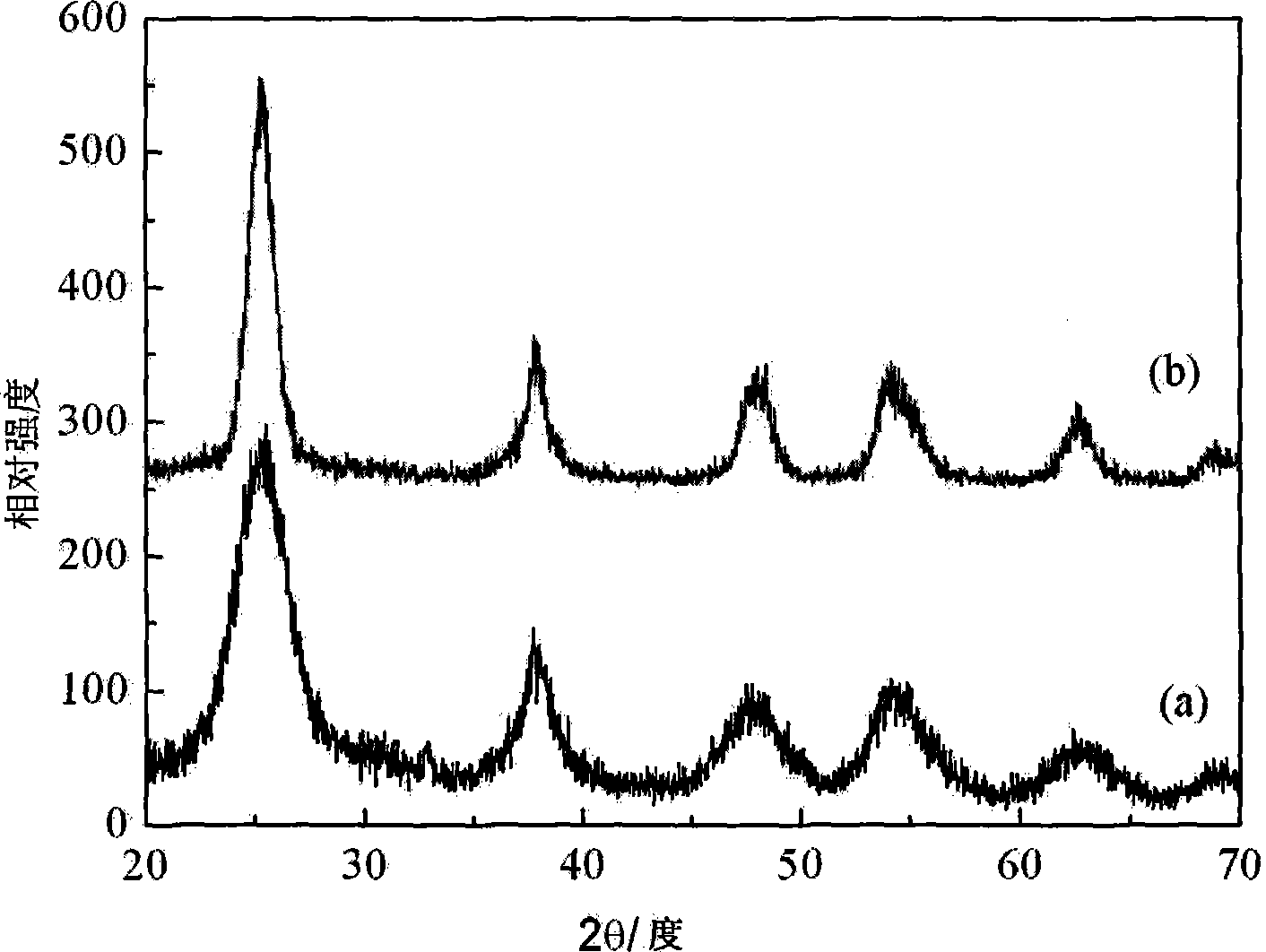
[0044] Add 145mL of 3mol / L ammonia water dropwise to 100mL of 1mol / L titanium tetrachloride solution at 70°C, keep it warm at 70°C for 12 hours, neutralize to a weakly alkaline pH value of about 8, and obtain a white precipitate, which is washed repeatedly with distilled water , to obtain a gel-like solid, get 40 grams of wet precipitation (converted into titanium dioxide to be 6 grams, XRD analysis shows that what obtained under such conditions is the mixed crystal form of anatase and rutile) and disperse it in 100 milliliters of nitric acid of 1mol / L In 80°C, the gel was degelized for 8 hours to obtain a light blue transparent titanium dioxide sol. The sol contained some white precipitates that could not be resolved. After standing still, the bottom precipitate was separated by centrifugation. XRD analysis showed that the bottom precipitate was rutile phase titanium dioxide, and the upper colloid appeared similar to figure 2 (b), XRD diffraction peaks that can be attributed...
Embodiment 3
[0048] After heating 100mL of 0.5mol / L titanium sulfate solution to 40°C, add dropwise 138mL of 1.5mol / L ammonia solution to the solution, keep it at 40°C for 20 hours, and neutralize until the pH value is approximately equal to 7 to obtain a white precipitate. Wash repeatedly to obtain a gel-like solid. Take 40 grams of wet precipitate (6.2 grams converted into titanium dioxide) and disperse it in 25 milliliters of 1 mol / L hydrochloric acid and 25 milliliters of 1 mol / L nitric acid mixed solution, degumming at 65 ° C After 5 hours, a light blue transparent sol was obtained, and the concentration of titanium dioxide was 0.94 mol / L. After diluting different times with deionized water, its UV-Vis absorption spectrum is similar to figure 1 , no absorption in the visible region. The powder obtained by vacuum drying was subjected to X-ray diffraction analysis, and the results showed that the titanium dioxide therein was an anatase phase, similar to figure 2 (b) shown.
[0049] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com