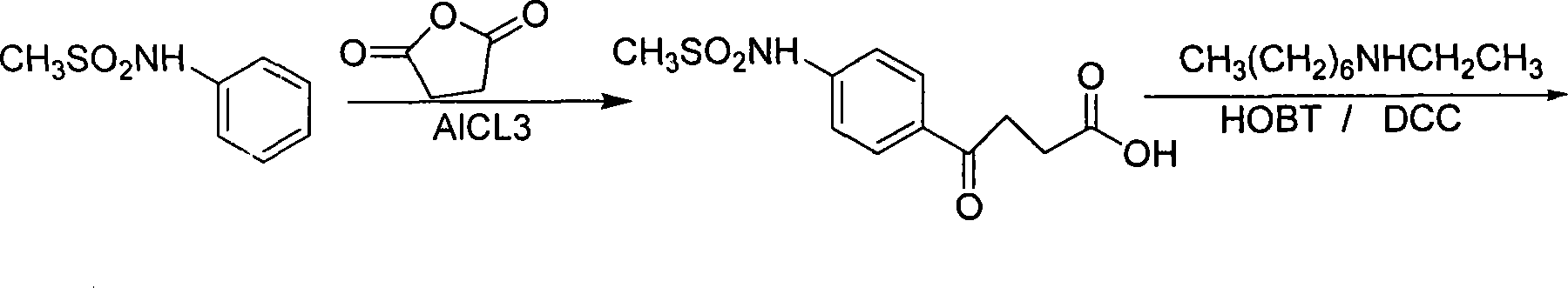
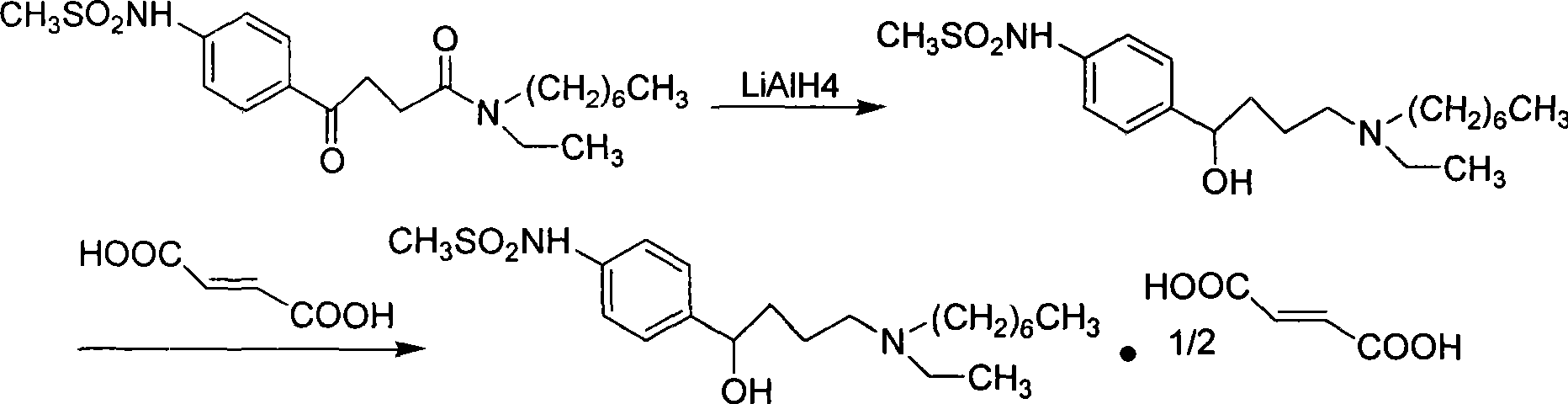
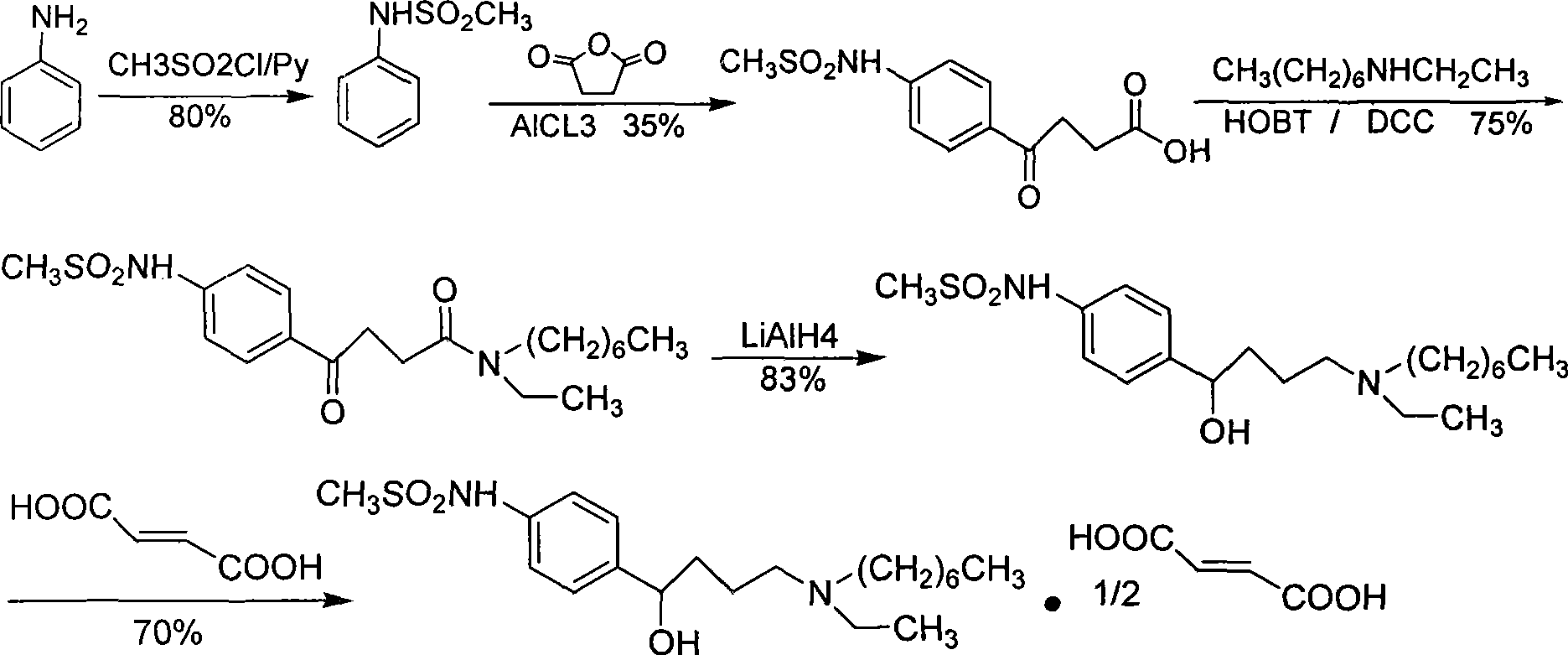
Ibutilide fumarate synthetic process
A synthesis process, a maleate technology, which is applied in the directions of drug combination, sulfonic acid amide preparation, cardiovascular system diseases, etc., can solve the problems of low yield, no industrial production of methanesulfonanilide, low total yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Preparation of N-ethyl-butyrolactam (product 2)
[0032]
[0033] Add 320ml of N,N-dimethylformamide (DMF) into a dry 1000ml three-necked flask, slowly add 28.8g (0.6mol) of 50% sodium hydride under ice water cooling, then slowly drop into 34.0g (0.4mol) ) a mixture of butyrolactam-80ml DMF. After the dropping, 43.6 g (0.4 mol) of ethyl bromide was slowly added dropwise. After the dropping, the mixture was stirred at room temperature for 2 hours. Slowly add 600ml of ice water dropwise under ice water cooling, extract with 200ml of dichloromethane x 2, wash with 200ml of water to obtain an organic layer, add 60g of sodium sulfate to dry. After filtering, the filter cake was washed with dichloromethane, and the filtrate was recovered under reduced pressure to exhaust the solvent, and 36.94 g of a light yellow residual liquid was obtained, which was the product (2), with a yield of 81.6%.
[0034] 2. Preparation of 4-ethylamino-n-butyric acid (product 3)
[0035] ...
Embodiment 2
[0066] 1, the preparation of N-ethyl-butyrolactam (product 2)
[0067] Add 80ml of DMF to a dry three-necked flask, slowly add 7.2g (0.15mol) of 50% sodium hydride under ice water cooling, and then slowly drop in a mixture of 8.5g (0.1mol) of butyrolactam-20ml of DMF. After the dropping, 9.68 g (0.15 mol) of ethyl chloride was slowly added dropwise. After the dropping, the mixture was stirred at room temperature for 2 hours. Slowly add 150ml of ice water dropwise under ice water cooling, extract with 50ml of dichloromethane x 2, wash with 50ml of water to obtain an organic layer, add 15g of sodium sulfate to dry. After filtration, the filtrate was recovered under reduced pressure to exhaust the solvent, and 13.81 g of the residual liquid was obtained, which was the product (2) N-ethyl-butyrolactam, with a yield of 81.3%.
[0068] 2, Preparation of 4-ethylamino-n-butyric acid (product 3)
[0069] Add 11.3 g (0.1 mol) of N-heptyl-butyrolactin prepared into a three-necked flask...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com