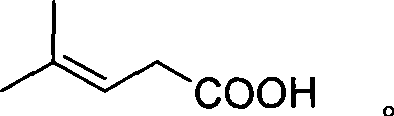
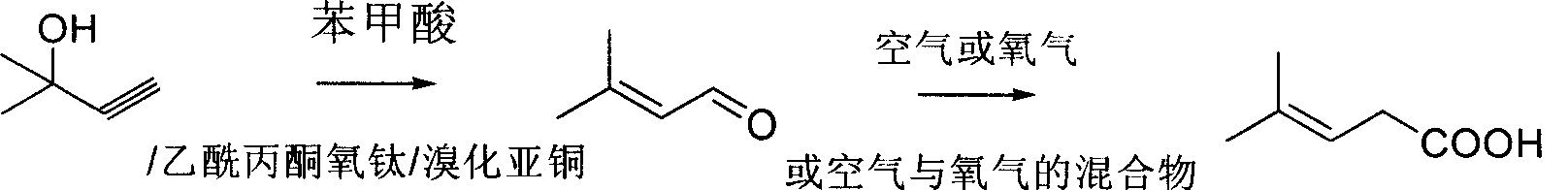
Method for preparing 3-methyl-2-butenoic acid
The technology of crotonic acid and methyl group is applied in the field of preparation of spice, medicine and pesticide intermediate 3-methyl-2-butenoic acid, and can solve the problems of large environmental pollution, high equipment requirements, production of sulfuric acid waste water and the like, To achieve the effects of environmental friendliness, high reaction yield and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 is the preparation of 3-methyl-2-butenal, and Embodiment 2-6 is the preparation of 3-methyl-2-butenoic acid.
[0019] Example 1
[0020] In the 500ml there-necked flask with mechanical stirring, thermometer, reflux condenser, drop into 95% 2-methyl-3-butyn-2-alcohol (water content 5%) 88.5g (1.0mol), liquid paraffin 100g, 6.1g (0.05mol) of benzoic acid, 2.6g (0.01mol) of titanyl acetylacetonate, 1.4g (0.01mol) of cuprous bromide, heated to 110-120°C for 3 hours under stirring, and cooled to room temperature after the reaction was completed. After filtration, the filtrate was rectified under reduced pressure to obtain 76 g of 3-methyl-2-butenal, with a yield of 90.5%.
Embodiment 2
[0022] In the 1000ml four-necked bottle with mechanical stirring, thermometer, vent pipe, condenser, drop into 420g (5.0mol) 3-methyl-2-butenal, drop into manganese acetate 0.5g, start stirring, keep stirring speed as 800-1000 rpm, under normal pressure, feed air from below the liquid surface at a rate of 1 liter / min, keep the reaction temperature at 30-40°C, feed the air for 24 hours, stop the air flow, and cool to 0°C , precipitated 3-methyl-2-butenoic acid solid, filtered, and dried to obtain 150g of 3-methyl-2-butenoic acid; 265g of the filtrate was recovered, and the filtrate contained 55g of 3-methyl-2-butenoic acid , 210 g of 3-methyl-2-butenal, and the total yield of 3-methyl-2-butenoic acid was 82%.
Embodiment 3
[0024] Put 420g (5.0mol) of 3-methyl-2-butenal in a 1000ml four-necked bottle equipped with a mechanical stirrer, a thermometer, a vent tube, and a condenser, and put in 0.5g of cuprous chloride, start stirring, and keep stirring The speed is 800-1000 rpm, and the air is passed under the liquid surface at a rate of 1 liter / min under normal pressure, and the reaction temperature is kept at 60-70°C, and the air is fed for 8 hours, and the air is stopped while it is hot. Filtrate, cool to 0°C, precipitate 3-methyl-2-butenoic acid solid, filter and dry to obtain 160g of 3-methyl-2-butenoic acid; recover 265g of filtrate, which contains 3-methyl-2-butenoic acid 35 g of 2-butenoic acid and 220 g of 3-methyl-2-butenal, the total yield of 3-methyl-2-butenoic acid was 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com