Method for measuring relative material of sodium divalproate
A technology of divalproex sodium and its determination method, which is applied in the field of drug detection, can solve problems such as unspecified substance inspection items and limits, clinical drug safety threats, etc., and achieve the effects of strong specificity, high sensitivity, and accurate detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
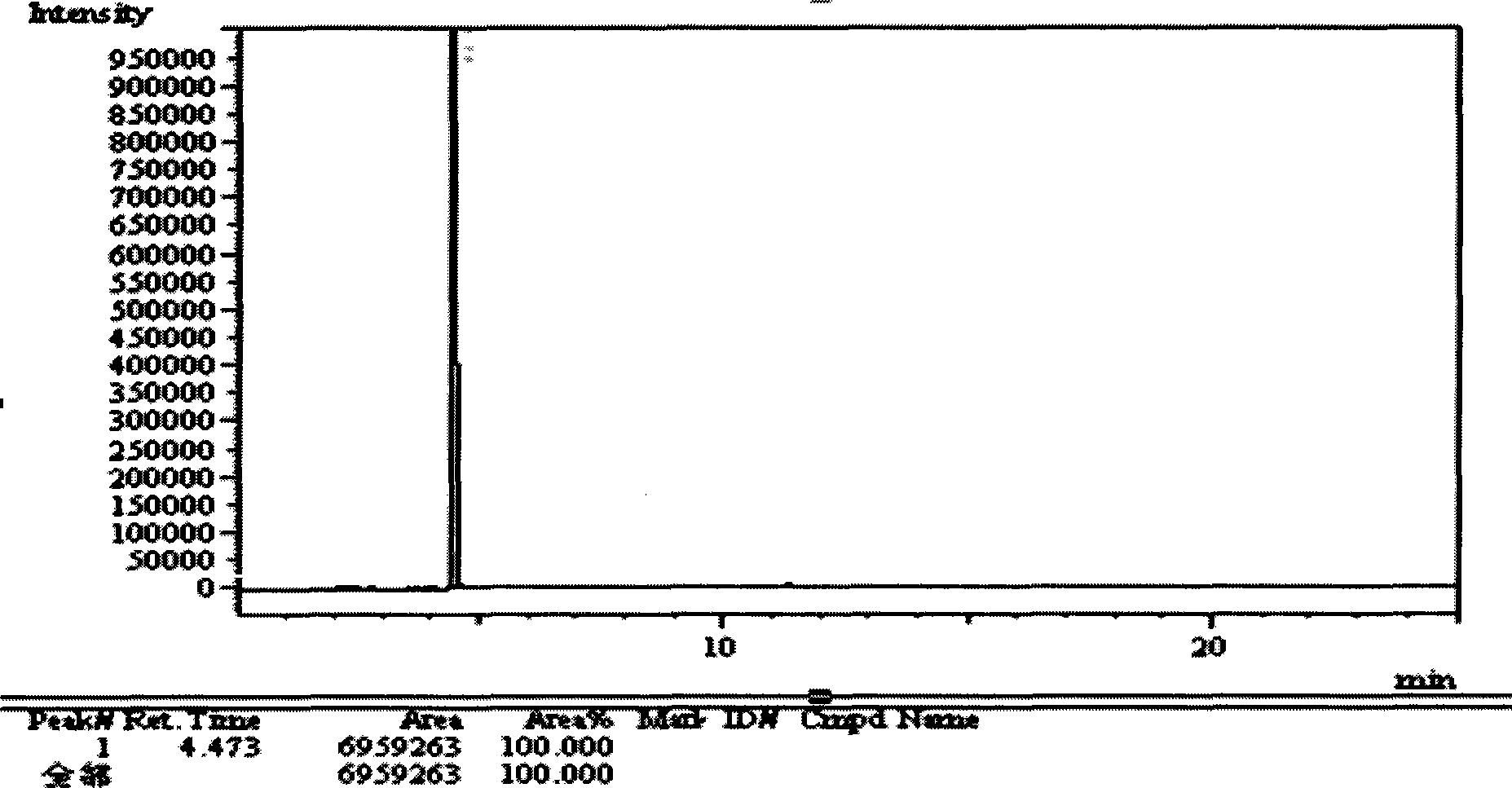
[0075] The preparation method of the divalproex sodium sample mainly includes the screening of two substances: the acid for acidifying the sample, and the organic solvent for extracting valproic acid. Taking the content of divalproex sodium, the measured amount of related substances and the separation situation in the determination process of related substances as investigation indicators, acid and organic solvents were screened and studied, as shown in Table 4:
[0076] Chromatographic column: DB-WAX capillary column 30m*0.25mm*0.25μm; inlet temperature: 260°C; column temperature: 145°C; carrier gas: helium.
[0077] Sample preparation: Take about 2g of divalproex sodium, add 50ml of water, heat to dissolve, let cool, add 1ml of acid, add 15ml of organic solvent, shake, and let it stand to separate layers. The organic solvent layer was taken, evaporated to dryness, and the remaining liquid was tested for diallyl acetic acid.
[0078] Table 4 Determination of acids and organi...
Embodiment 1
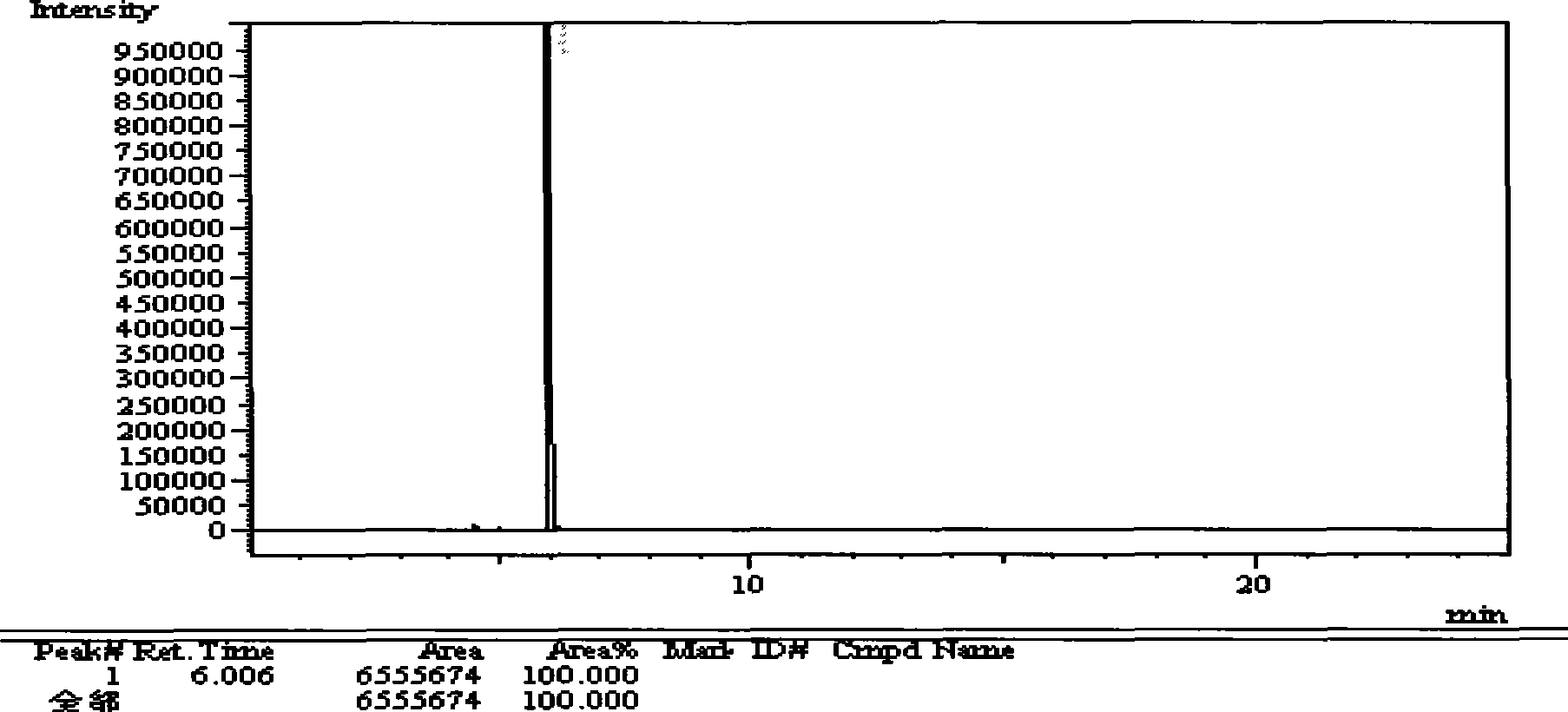
[0109] (1) Chromatographic conditions
[0110] AE·PEG-20M capillary column 30m*0.25mm*0.25μm.
[0111] Injection port temperature: 240°C; column temperature: 130°C; carrier gas: hydrogen.
[0112] (2) Sample preparation
[0113] Take about 1g of divalproex sodium, add 30ml of water, heat to dissolve, let it cool, add 3ml of dilute hydrochloric acid, add 10ml of cyclohexane, shake, let it stand to make layers. Take the cyclohexane layer, evaporate it to dryness, and leave a residual liquid.
[0114] (3) Determination method
[0115] Precisely measure 0.1 μl of the residual liquid, inject it into a gas chromatograph, and record the chromatogram up to twice the retention time of the main component peak. If there are impurity peaks in the spectrogram of the test product, the sum of the areas of each impurity peak must not exceed 3% of the total area.
Embodiment 2
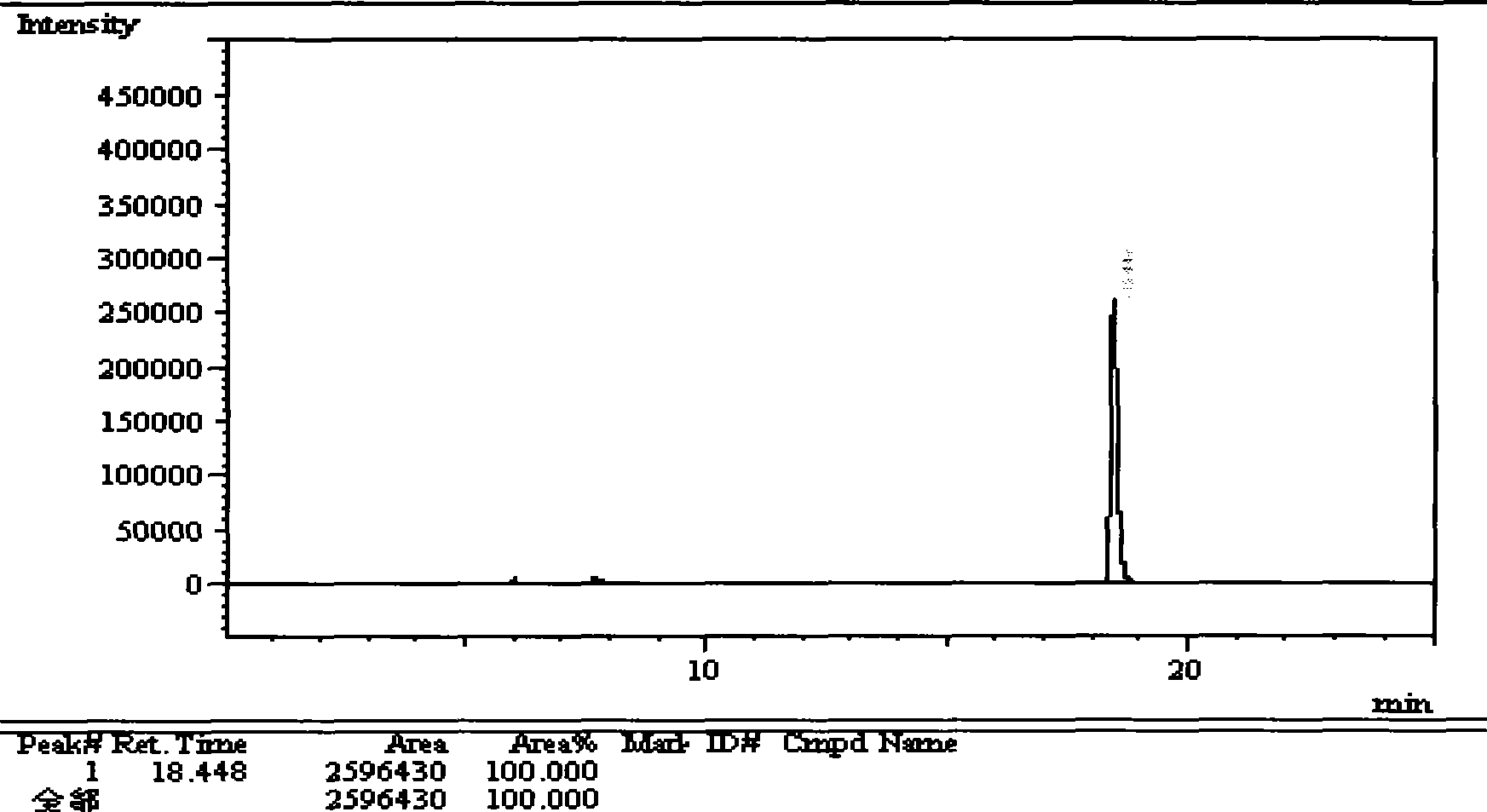
[0117] (1) Chromatographic conditions
[0118] DB-WAX capillary column 30m*0.25mm*0.25μm.
[0119] Injection port temperature: 220°C; column temperature: 150°C; carrier gas: nitrogen.
[0120] (2) Sample preparation
[0121] Take about 5g of divalproex sodium, add 100ml of water, heat to dissolve, let cool, add 2.5ml of phosphoric acid solution, add 35ml of petroleum ether, shake, let stand to make layers. Take petroleum ether, evaporate to dryness, remaining liquid.
[0122] (3) Determination method
[0123] Precisely measure 0.1 μl of the residual liquid, inject it into a gas chromatograph, and record the chromatogram up to twice the retention time of the main component peak. If there are impurity peaks in the spectrogram, the sum of the areas of each impurity peak shall not exceed 3% of the total area.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com