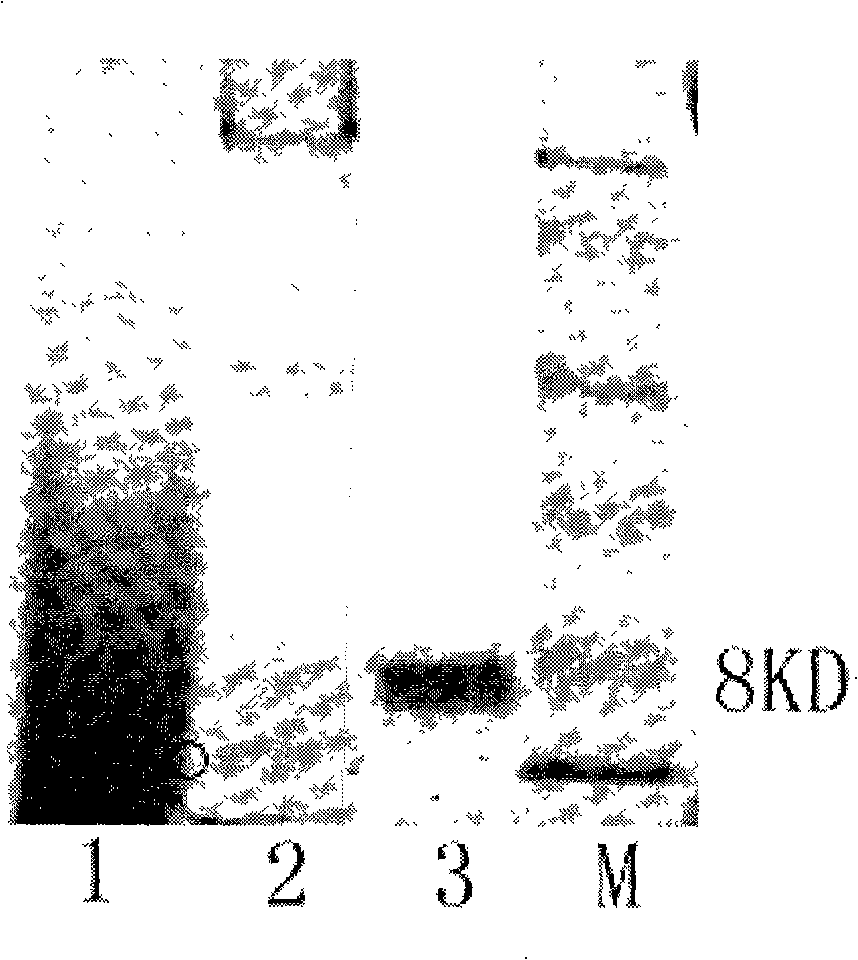Heterologous expression and purification method for human RANTES protein having chemotaxis
A technology of heterologous expression and purification method, applied in the field of bioengineering pharmacy, can solve the problems of low protein activity, imperfect post-translational processing mechanism of protein expression, unfavorable research work, etc. The effect of easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
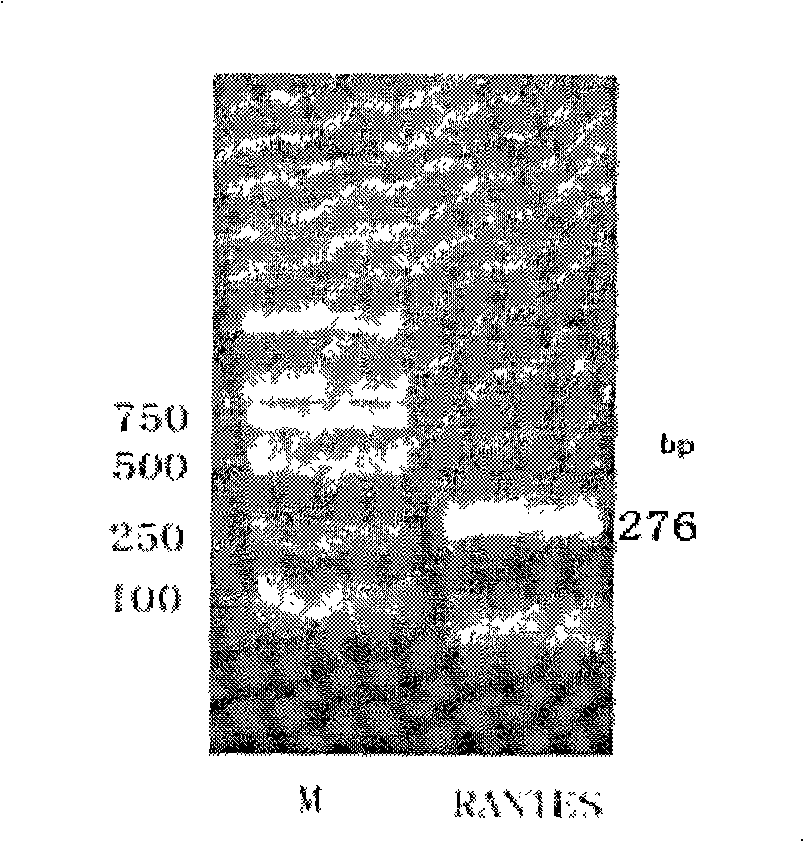
[0074] Embodiment 1: Cloning of human RANTES gene
[0075] According to the reported human RANTES gene sequence (NCBI accession number: NM 002985), two primers F1 (SEQ ID NO: 3) and R1 (SEQ ID NO: 4) were designed, RNA was extracted from human blood, and human RANTES was amplified by RT-PCR Gene fragment. The primer sequences are as follows:
[0076] Upstream primer F1: 5'----ATGAAGGTCTCCGCGGCAGCCC----3'
[0077] Downstream primer R1: 5'----CTAGCTCATCTCCAAAGAGTTGATG----3'
[0078] The reverse transcription system is 20ul, which contains 2ul of 10×RT reaction buffer, 25mM MgCl 2 4ul, 10mMdNTP 2ul, 200U / ul RNase Inhibitor 0.5ul, AMV Reverse Transcriptase 1ul, downstream primer R1 (20μM) 1ul, total RNA (2μg / μl) 2ul. The reaction conditions were: incubate at 42°C for 60 minutes, heat at 80°C for 5 minutes, and cool on ice for 5 minutes. Then, PCR amplification was carried out using the above-mentioned reverse transcription product cDNA as a template. The PCR reaction system...
Embodiment 2
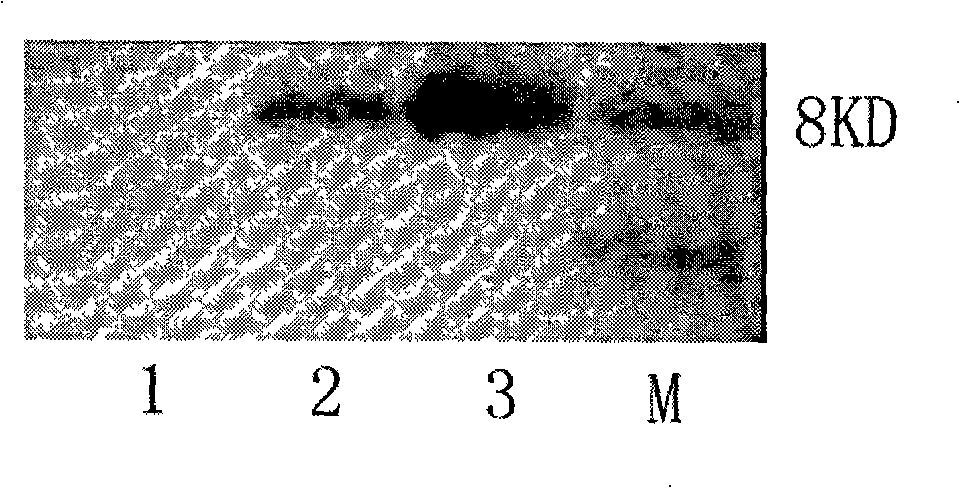
[0079] Embodiment 2: Construction of human RANTES fusion protein expression vector
[0080] In order to facilitate the separation and purification of human RANTES protein, a 6×His purification tag was fused to the N-terminus of the target protein, and an enterokinase (EK) cleavage site was added between the His-tag and RANTES protein to construct a human RANTES fusion protein. For this purpose, a pair of primers F2 and R2 were designed, and the primer sequences are as follows:
[0081] F2: 5'----GCGAATTC CATCATCATCATCATCAT GATGACGATGACAAG ATGAAGGTC----3'
[0082] R2: 5'----GC GCGGCCGC CTA GCT CAT CTC CAA AGA GT----3'
[0083] At the 5' end of the upstream primer F2, an EcoR I restriction site, a DNA sequence of 6 histidines and an enterokinase recognition site (Asp-Asp-Asp-Asp-Lys) (SEQ ID NO : 6), a NotI restriction site (SEQ ID NO: 7) was added to the 5' end of the downstream primer R2, and then the human RANTES fusion protein gene fragment (SEQ ID NO: 7) was amplified by ...
Embodiment 3
[0084] Embodiment 3: Construction of human RANTES fusion protein engineered yeast
[0085] pPIC9K is an integrative vector that can integrate foreign genes into the chromosomes of Pichia pastoris cells through homologous recombination, and can achieve multi-site integration to obtain multiple copies. Generally speaking, the expression level and copy number of foreign proteins Positive correlation. Since pPIC9K contains the Kanamycin (Kan) gene that can resist G418, the Kan gene and the foreign gene will be integrated into the chromosome together during transformation, so that the transformant has G418 resistance, and the resistance level is positively correlated with the number of Kan genes. Therefore, we obtained high-expression recombinant transformants through G418 resistance screening of transformants. The screened transformants also need to identify the phenotype, that is, to determine whether they are Mut+ or Muts. Transformants can be spotted on both MD and MM plates ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com