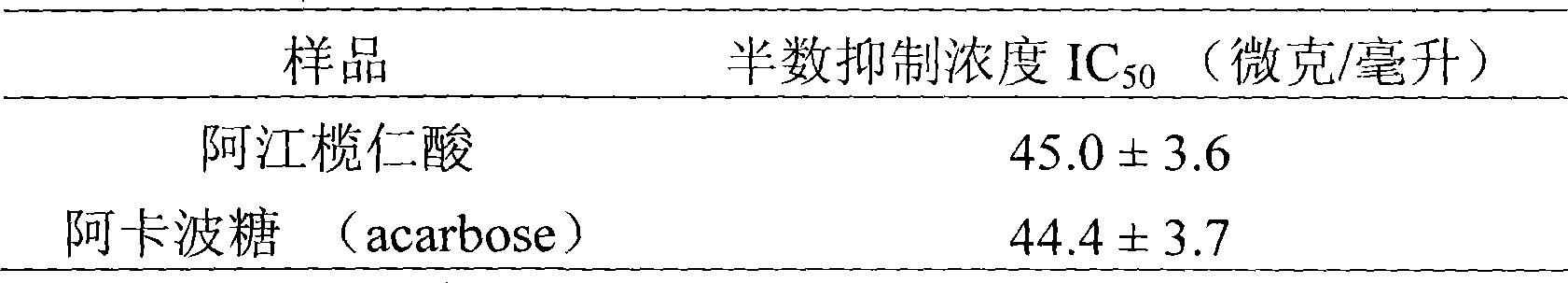
Use of arjunolic acid in preparing glycosidase inhibitor
A technology of glycosidase inhibitor, terminalisoleic acid, which is applied in the field of natural medicinal chemistry to achieve the effects of low pollution, convenient extraction and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 : Preparation of arjunolic acid from dried leaves of Lagerstroemia tomentosa
[0018] 1.1 Instruments and reagents
[0019] H NMR spectrum ( 1 H-NMR), carbon nuclear magnetic resonance spectrum ( 13 C-NMR) and two-dimensional nuclear magnetic resonance spectrum (2D NMR) were determined by INOVA type superconducting nuclear magnetic resonance spectrometer (VARIAN INOVA-400MHz) (tetramethylsilyl ether was internal standard); electrospray mass spectrometry (ESI-MS) was determined by Bruker Esquire 3000+ mass spectrometer, silica gel (100-200, 200-300) for column chromatography and silica gel GF254 (10-40 mesh) for thin layer chromatography were purchased from Qingdao Ocean Chemical Factory; all reagents used were of analytical grade , wherein the boiling range of petroleum ether is 60-90°C; thin plate (TLC) detection with 254nm and 365nm ultraviolet lamps; chromogen with 10% sulfuric acid-ethanol and bromocresol green solution.
[0020] 1.2 Plant source and i...
Embodiment 2
[0028] Example 2 : Preparation of arjunolic acid from fresh leaves of Lagerstroemia tomentosa
[0029] 2.1 Instruments and reagents: Same as Example 1.
[0030] 2.2 Plant source and identification: Same as Example 1.
[0031] 2.3 Extraction and separation
[0032] The sample (0.5 kg of fresh leaf weight) was immediately minced with a knife and extracted twice at room temperature with 95% ethanol for 24 hours each time. The extracts were cooled and combined, and concentrated under reduced pressure to obtain 35 grams of brown-black viscous crude extract. The crude extract was dissolved in 2 liters of hot water, degreased with petroleum ether (2 liters / time, 3 times in total), then extracted with ethyl acetate (2 liters / time, 5 times in total), and the solvent was evaporated under reduced pressure, The obtained ethyl acetate extract (8.3 grams) was mixed with 10 grams of 100-200 mesh silica gel, and was subjected to column chromatography on a silica gel column (200-300 mesh,...
Embodiment 3
[0034] Example 3 : Preparation of arjunolic acid from stems and root bark of Lagerstroemia tomentosa
[0035] 3.1 Instruments and reagents: Same as Example 1.
[0036] 3.2 Plant source and identification: Same as Example 1.
[0037] 3.3 Extraction and separation
[0038] The sample (0.5 kg dry weight of stems, branches and root bark) was pulverized and extracted twice at room temperature with 95% ethanol for 24 hours each time. things. The crude extract was dissolved in 2 liters of hot water, degreased with petroleum ether (2 liters / time, 3 times in total), then extracted with ethyl acetate (2 liters / time, 5 times in total), and the solvent was evaporated under reduced pressure, The obtained ethyl acetate extract (9.6 grams) was mixed with 10 grams of 100-200 mesh silica gel, and was subjected to column chromatography on a silica gel column (200-300 mesh, 150 grams), and was mixed with petroleum ether-acetone (100:0-0: 100) is the gradient elution of the eluent, and ever...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com