P-benzene dicarboxylic acid hydrogen refining catalyst and preparation method thereof
A technology for terephthalic acid and hydrorefining, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of poor sulfur resistance and easy growth of palladium crystal grains.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Get 50 grams of granular coconut shell charcoal carriers for pretreatment, soak in 400 milliliters of concentration in a dilute nitric acid solution of 0.08 (weight) % for 4 hours, then wash with deionized water until neutral, drain, and dry at 105 ° C 24 hours, naturally cooled to room temperature.
[0032] Dissolve chloropalladium acid, lanthanum nitrate and tartaric acid in 20 milliliters of deionized water, adjust the pH value of the solution to 3.0 with 8 (weight)% sodium carbonate, and finally set the volume to 40 milliliters to obtain palladium glue.
[0033] The carrier is put into a rotating pan, and the prepared palladium glue solution is sprayed on the carrier to prepare a catalyst precursor. The catalyst precursor was left to age for 10 hours, then reduced with hydrogen at 200 °C for 6 hours, cooled naturally to room temperature under a hydrogen atmosphere, and finally washed with pure water to be free of Cl - So far, the catalyst of the present invention i...
Embodiment 2
[0035] Get 50 grams of granular coconut shell charcoal carriers for pretreatment, soak in 400 milliliters of concentration in 0.1 (weight) % dilute hydrochloric acid solution for 4 hours, then wash with deionized water until neutral, drain, and dry at 105 ° C 24 hours, naturally cooled to room temperature.
[0036] Palladium acetate, lanthanum nitrate, and tartaric acid were dissolved in 20 milliliters of deionized water, and the pH value of the solution was adjusted to 4.0 with 8 (weight)% sodium carbonate, and finally the solution was constant to 40 milliliters to obtain palladium glue.
[0037] The carrier is put into a rotating pan, and the prepared palladium glue solution is sprayed on the carrier to prepare a catalyst precursor.
[0038] Place the catalyst precursor for aging for 24 hours, prepare a reduction solution with 20 grams of 5 (weight)% hydrazine hydrate and 200 grams of pure water, soak the catalyst precursor in the reduction solution for 2 to 4 hours, and fin...
Embodiment 3~6
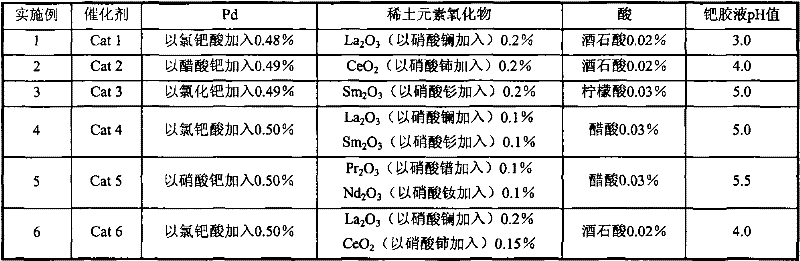
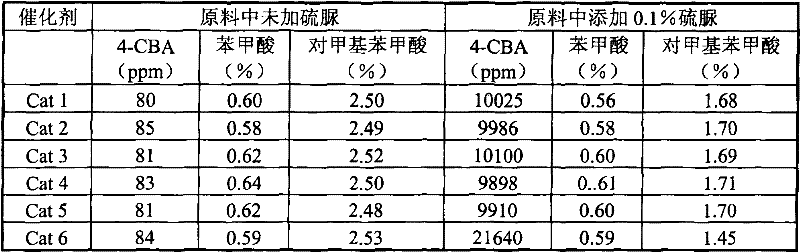
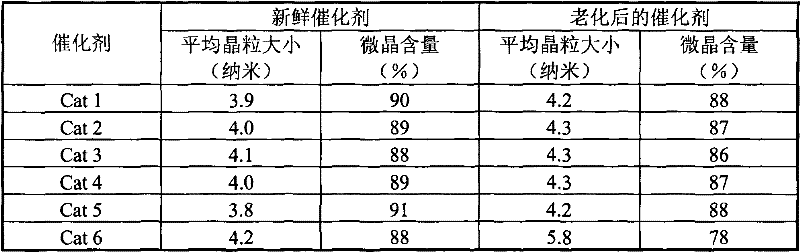
[0040] Catalysts Cat 3-Cat 6 were prepared by the same method as [Example 1]. See Table 1 for the weight percent composition of each component of the catalyst and the pH value of the palladium colloid solution.
[0041] Table 1
[0042]
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com