Low-temperature sulfur-tolerant denitration catalyst and preparation method and application thereof
A denitrification catalyst and catalyst technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of high preparation cost of low-temperature water resistance and sulfur resistance, high low temperature activity, low water resistance and sulfur resistance, etc. , achieve good thermal stability and mechanical properties, good low-temperature activity and sulfur resistance, improve dispersion and water resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
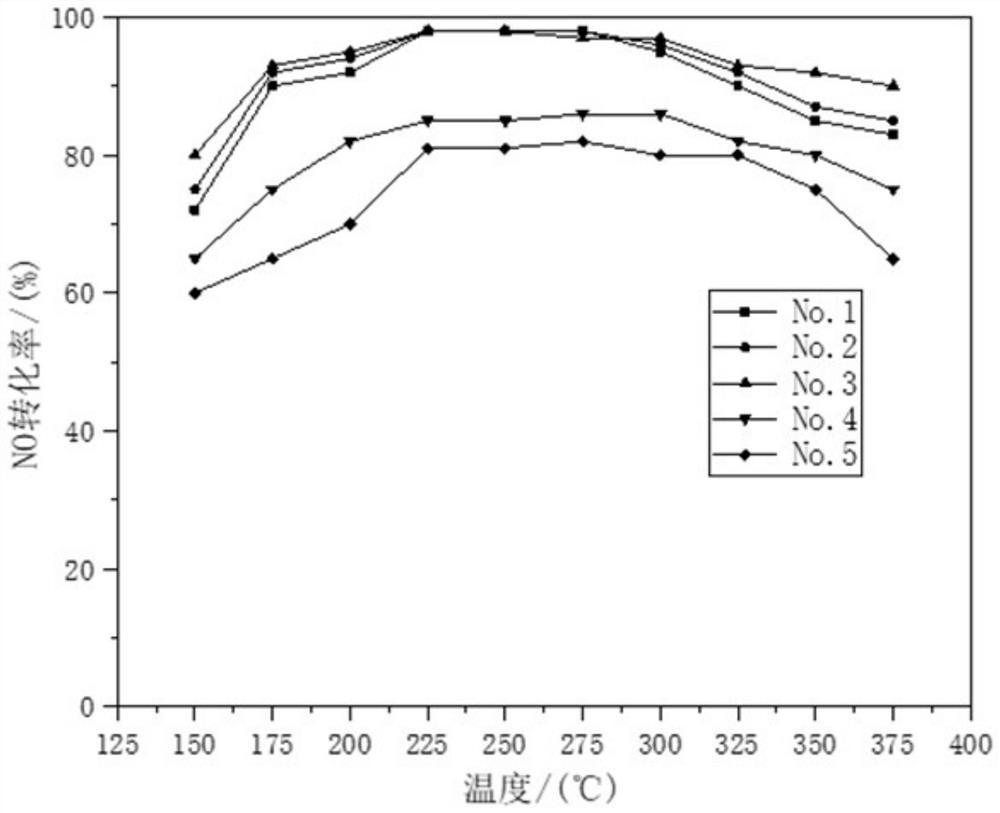
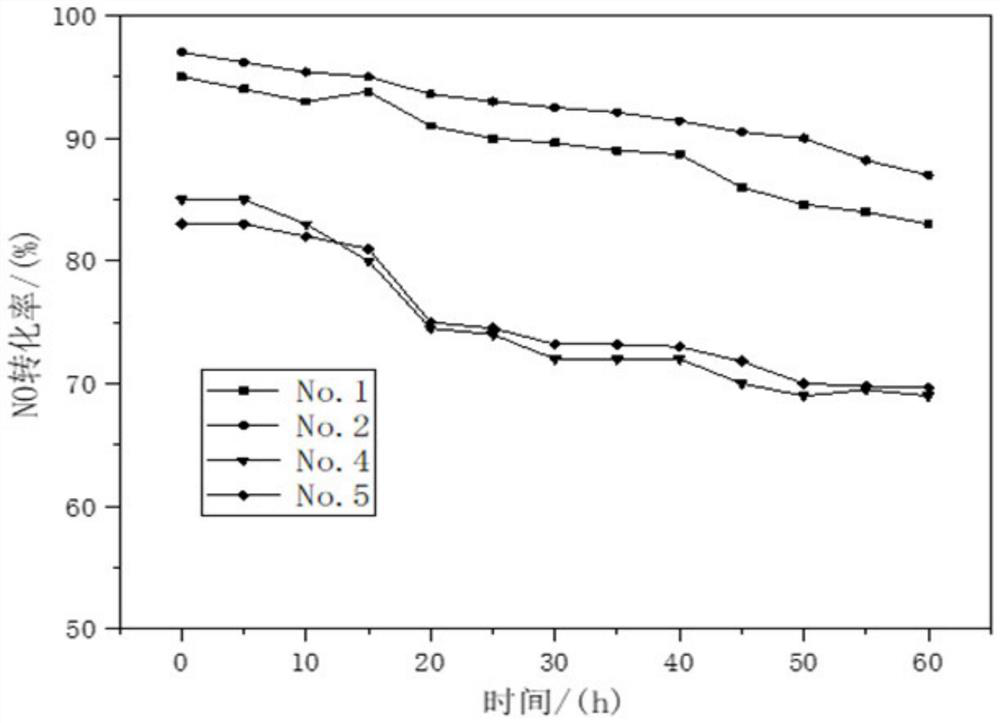
[0034] (1) The carrier is prepared by co-precipitation method, and 6.34g Zr(NO 3 ) 4 ·5H 2 O, 2.75gFe(NO 3 ) 3 9H 2 O and 5.34g of metatitanic acid powder were dissolved in water and mixed evenly, then according to Fe 3+ with Zr 4+ Add 1-2 times of the theoretical amount of precipitant required for complete precipitation of ions, including ammonia water, to form a precipitate. After stirring and aging at 40° C. for 4 h, the product was filtered and washed to form a carrier precursor.
[0035] (2) The perovskite catalyst was prepared by the chemical complexation method, and 43.3g La(NO 3 ) 2 ·6H 2 O, 2.9gNi(NO 3 ) 2 ·6H 2 O, 0.291gCo(NO 3 ) 2 ·6H 2 O and 22.34gMn(NO 3 ) 2 4H 2 O was dissolved in water, and 53.46g of ammonium citrate complexing agent was added to form a uniform mixed solution. Then add the carrier precursor obtained in step (1), mix to form a uniform gel, dry the gel at 100°C for 12 hours, and then bake the gel at 350°C for 4 hours to obtain t...
Embodiment 2
[0039] (1) The carrier is prepared by co-precipitation method, and 3.972g Zr(NO 3 ) 4 ·5H 2 O, 1.725gFe(NO 3 ) 3 9H 2 O and 3.349g metatitanic acid powder were dissolved in water and mixed evenly, then according to Fe 3+ with Zr 4+ Add 1-2 times of the theoretical amount of precipitant required for complete precipitation of ions, including ammonia water, to form a precipitate. After stirring and aging at 70° C. for 4 h, the product was filtered and washed to form a carrier precursor.
[0040] (2) The perovskite catalyst was prepared by the chemical complexation method, and 43.3g La(NO 3 ) 2 ·6H 2 O, 5.82gNi(NO 3 ) 2 ·6H 2 O, 0.873gCo(NO 3 ) 2 ·6H 2 O and 19.327gMn(NO 3 ) 2 4H 2 O was dissolved in water, and 53.46g of ammonium citrate complexing agent was added to form a uniform mixed solution. Then add the carrier precursor obtained in step (1), mix to form a uniform gel, dry the gel at 100°C for 12 hours, and then bake the gel at 350°C for 4 hours to obtain...
Embodiment 3
[0044] (1) The carrier is prepared by co-precipitation method, and 12.38g Zr(NO 3 ) 4 ·5H 2 O, 5.374gFe(NO 3 ) 3 9H 2 O and 10.44g metatitanic acid powder were dissolved in water and mixed evenly, then according to Fe 3+ with Zr 4+ Add 1-2 times of the theoretical amount of precipitant required for complete precipitation of ions, including ammonia water, to form a precipitate. After stirring and aging at 80° C. for 4 h, the product was filtered and washed to form a carrier precursor.
[0045] (2) The perovskite catalyst was prepared by the chemical complexation method, and 43.3g La(NO 3 ) 2 ·6H 2 O, 8.72gNi(NO 3 ) 2 ·6H 2 O, 1.164gCo(NO 3 ) 2 ·6H 2 O and 16.57gMn(NO 3 ) 2 4H 2 O was dissolved in water, and 53.46g of ammonium citrate complexing agent was added to form a uniform mixed solution. Then add the carrier precursor obtained in step (1), mix to form a uniform gel, dry the gel at 100°C for 12 hours, and then bake it at 550°C for 4 hours to obtain the l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com