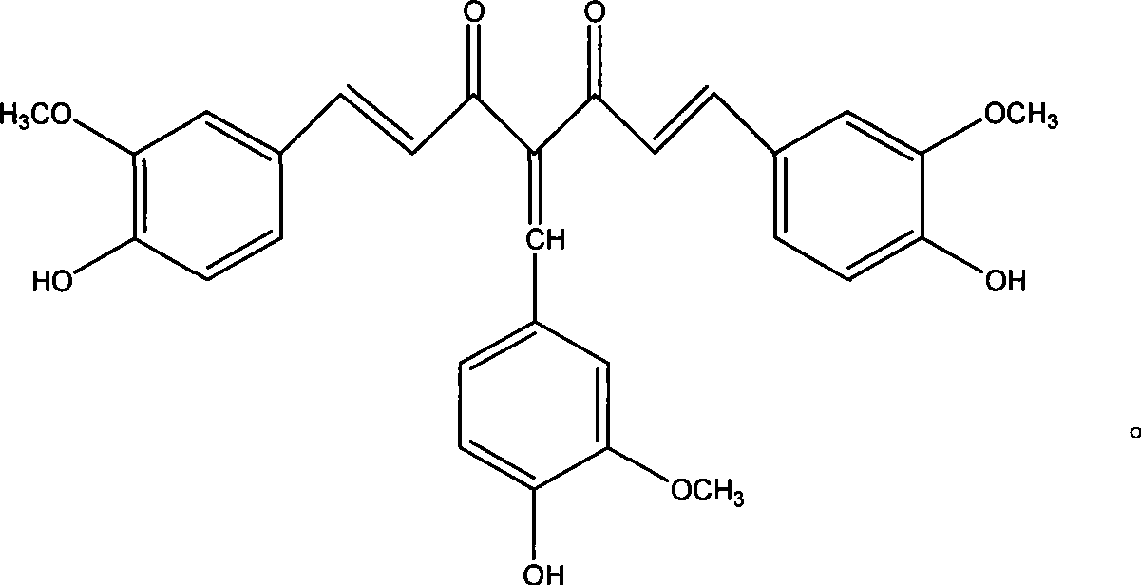
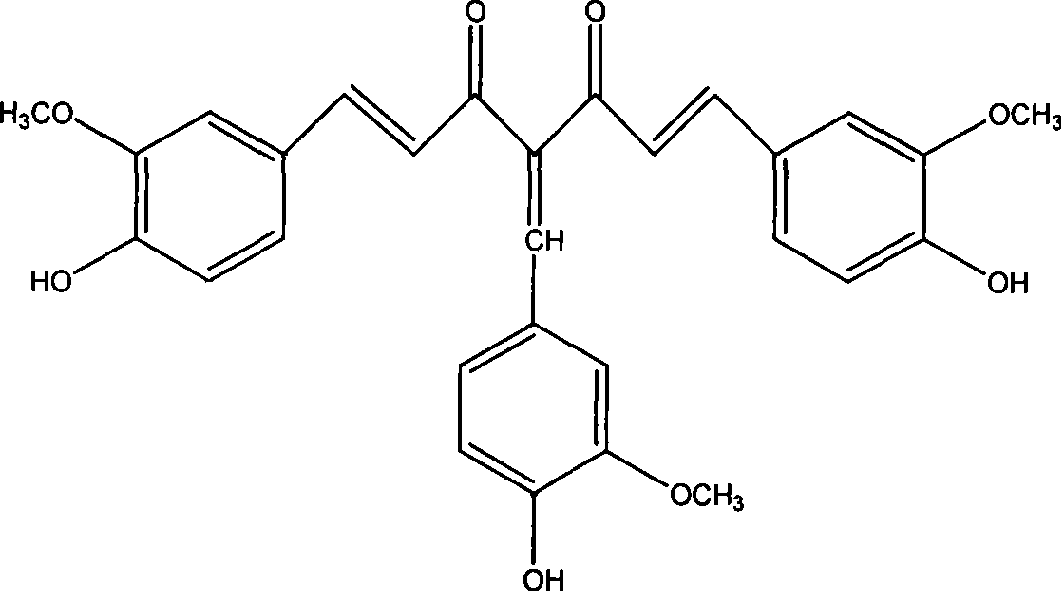
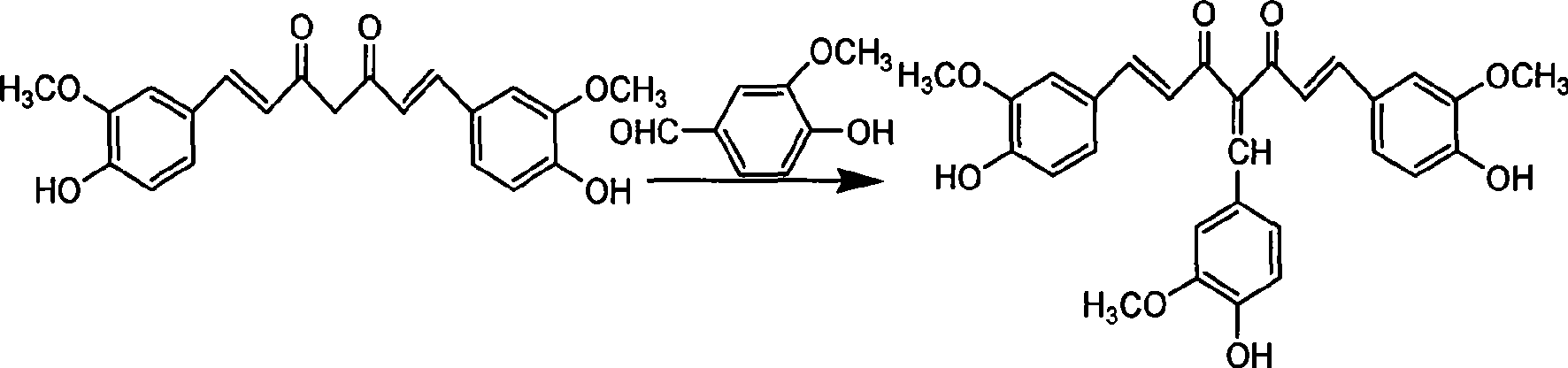
4-(4-hydroxy-3-methoxybenzene methylene) curcumin, preparation thereof and use in preparing anti-cancer medicament
A kind of technology of methoxybenzylidene and curcumin, applied in the field of synthesizing 4-curcumin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 Synthesis of 4-(4-hydroxy-3-methoxybenzylidene) curcumin.
[0017] Synthetic raw materials curcumin, 3-hydroxy-4-methoxybenzaldehyde (vanillin), and piperidine are from Sinopharm Chemical Reagent Co., Ltd., and all reagents are of analytical grade. Nuclear Magnetic Resonance Spectrometer (mercury-300, Varian Company of the United States) Ion Trap Mass Spectrometer (DECAX-30000, Thermo Finnigan Company of the United States); Micro-melting Point Apparatus (X-4, Shanghai Precision Instrument Factory).
[0018] Curcumin 2.2g (6mmol), methanol 50ml, add a catalytic amount of piperidine, vanillin 0.91g (6mmol), stir and react at room temperature for 48 hours, concentrate and remove the solvent, and the residue is separated and purified by silica gel column chromatography. The eluent is Ethyl acetate:petroleum ether=1:3 to obtain 0.9 g of reddish-brown powder (30% yield). mp96-98℃, molecular formula C 29 h 26 o 8 , 1 H NMR (300MHz, DMSO): δ (ppm) 3.72 (s, 3H, OC...
Embodiment 2
[0019] Example 2 4-(4-hydroxy-3-methoxybenzylidene) curcumin inhibits tumor cells K562, HL-60, B-16, SW480, HepG2, MGC80-3, SH-SY5Y, Bxpc-3 In vitro growth activity of:
[0020] 2.1. Cell lines
[0021] K562: Human chronic myelogenous leukemia blast cell line
[0022] HL-60: Human acute myeloid leukemia cell line
[0023] B16: mouse melanoma B16 cell line.
[0024] SW480: Human colon carcinoma cells
[0025] HepG2: human liver tumor cells
[0026] MGC80-3: human gastric cancer cells
[0027] SH-SY5Y: Human neuroblastoma cells
[0028] Bxpc-3: human pancreatic cancer cells
[0029] The above cells were all obtained from the Shanghai Cell Bank of the Chinese Academy of Sciences.
[0030] 2.2 Cell culture
[0031] See Table 1 for the formula of the cell culture medium, and the cells were kept at 37°C, 5% CO 2 Cultured in an incubator, and the cells in the logarithmic growth phase were used for proliferation and apoptosis experiments.
[0032] Table 1 Culture medium for...
Embodiment 3
[0042] Example 3 Inhibitory effect of intraperitoneal injection on B16 melanoma
[0043] 3.1 Materials BALB / C mice, aged 6-8wk, female, weighing 18g, were provided by the Shanghai Experimental Animal Center of the Chinese Academy of Sciences. Mouse melanoma B16 cell line. 4-(4-Hydroxy-3-methoxybenzylidene) curcumin is made into a solid dispersion, which is dissolved into a required concentration with physiological saline before use.
[0044] 3.2 Method
[0045] 3.2.1 Establishment of tumor-bearing mouse model B16 tumor cells were passed on for more than two generations, and the number of cells was adjusted to 10 7 / ml, 0.2mL / mouse, inoculate subcutaneously in the right forelimb of mice, inoculate 2 mice, wait for about two weeks, peel off the tumor, homogenate, and inoculate 30 mice again.
[0046] 3.2.2 Grouping were divided into 2 groups: the mice 24 hours after the inoculation of the tumor strain were randomly divided into 2 groups: Group I was the normal saline group (i.e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com