Detection method for cis-isomer in vitamin K2 medicine
A cis-isomer and detection method technology, applied in the field of detection of vitamin K2 drug impurities, can solve the problems of low precision, inability to accurately control the content of cis-isomer, poor specificity, etc., achieve high sensitivity, facilitate Promotional use, good specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
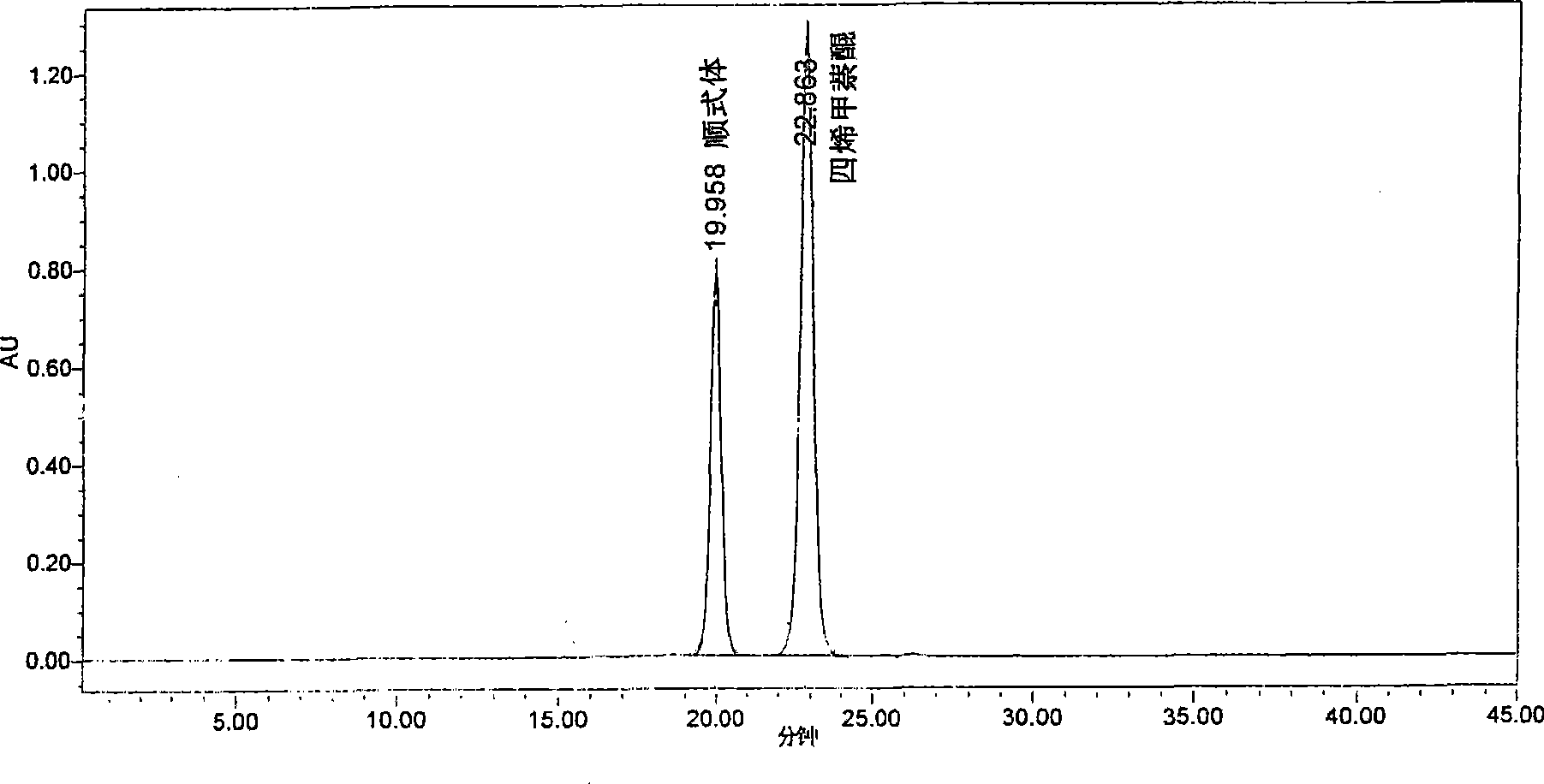
[0015] HPLC analysis conditions: HPLC analysis adopts high performance liquid chromatograph Waters 2695_996 equipped with diode array detector, and Agilent Prep-SIL (5μm4.6×250mm) silica gel column of Agilent Company. The mobile phase was n-hexane-n-butyl ether (volume ratio 95:5), the detection wavelength was 270nm, and an autosampler was used to inject 20 μl each time.
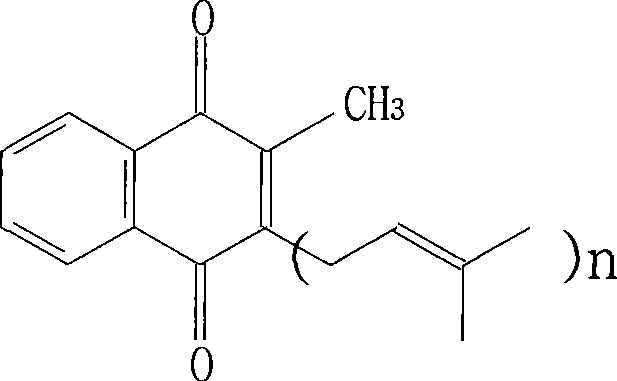
[0016] Respectively take an appropriate amount of menadione trans isomer and cis isomer, accurately weigh, and use the above-mentioned mobile phase to prepare a solution with a concentration of 1 mg / ml, analyze using the above-mentioned instruments and conditions, and obtain the HPLC chromatogram, Such as figure 1 shown. Under this chromatographic condition, the retention time of the trans isomer of menadione is 22.863min, the retention time of the cis isomer of menadione is 19.958min, and the separation degree of the two is greater than 1.5, which can reach Baseline separation.
Embodiment 2
[0018] Get 3 parts of menadione products as samples, analyze according to the HPLC analysis conditions in Example 1, calculate according to the cis-isomer peak area and the contrast solution peak area in the liquid chromatogram obtained, by external standard method Content of cis isomer. Table 1 shows the test results of the three samples. As can be seen from Table 1, there is little difference in the cis-isomer content in them, but they are all lower than the detection limit (2% ).
[0019] Table 1 Content of cis isomer in samples
[0020]
Embodiment 3
[0022] Take a 1mg / ml menadione sample solution, add hydrochloric acid solution, sodium hydroxide solution, and hydrogen peroxide solution respectively to carry out acid, alkali, and oxidative destruction reactions. Take 1mg / ml menatetrenone sample solution, heat in a water bath, and carry out thermal destruction reaction. Take a 1mg / ml menadione sample solution and expose it to light for several hours to carry out photodestruction reaction. The above reaction solution was analyzed using the HPLC analysis conditions in Experimental Example 1.
[0023] The purpose of this test is to investigate the sensitivity of the sample to acid, alkali, oxidation, heat, light and the content change of the cis-isomer, and then determine whether the chromatographic conditions used can accurately detect the content of the cis-isomer so as to fully control the product quality. The quality and test results are shown in Table 2. It can be seen from the results that the menatetrenone solution is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com