Bionic synthesizing method for chemosterilant
The technology of biomimetic synthesis and synthesis method is applied in the field of biomimetic synthesis of sterile agent gossypol, can solve the problems of inability to obtain industrialized production, low yield and the like, and achieves good economic benefits, development prospects, and yield improvement effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
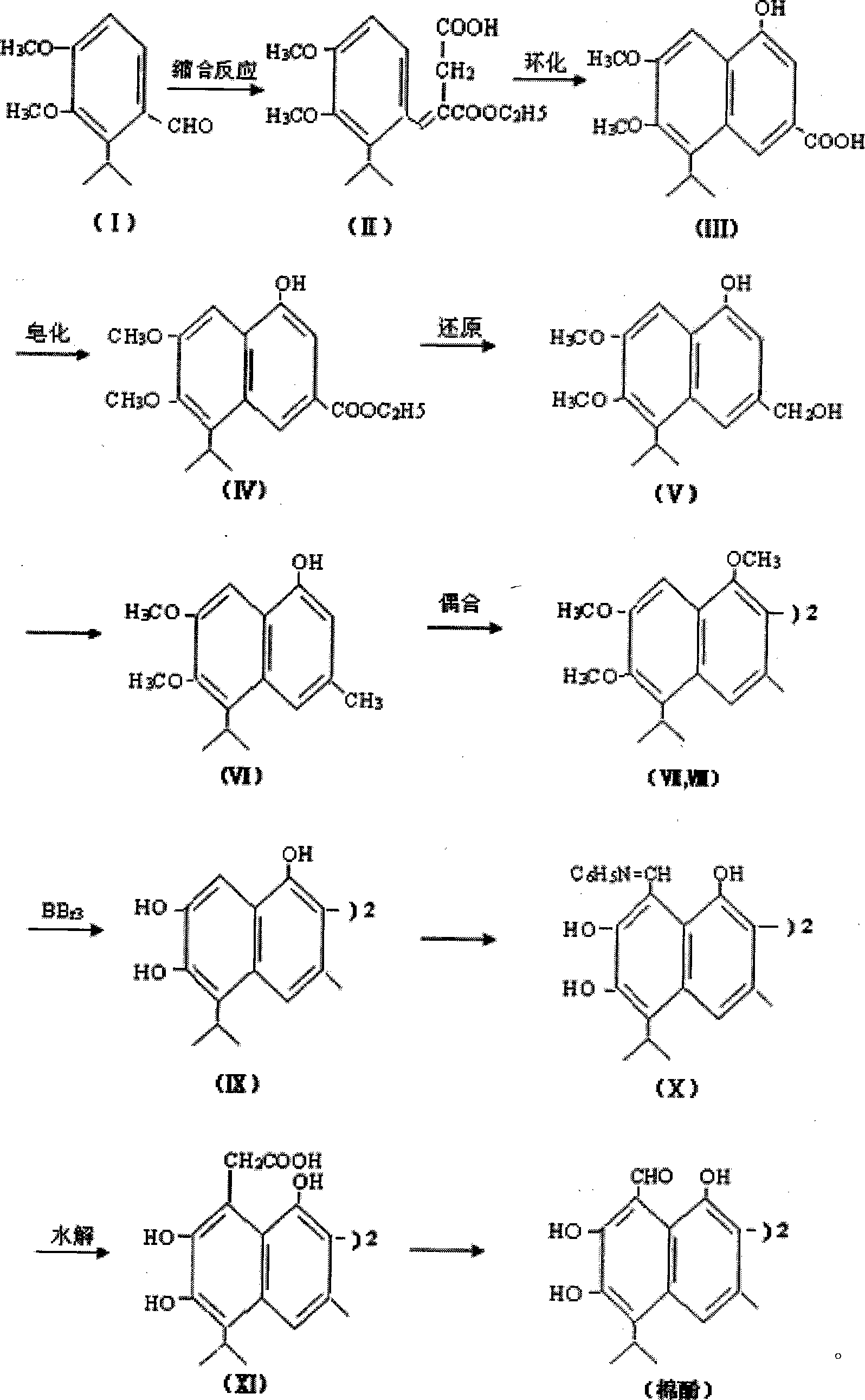
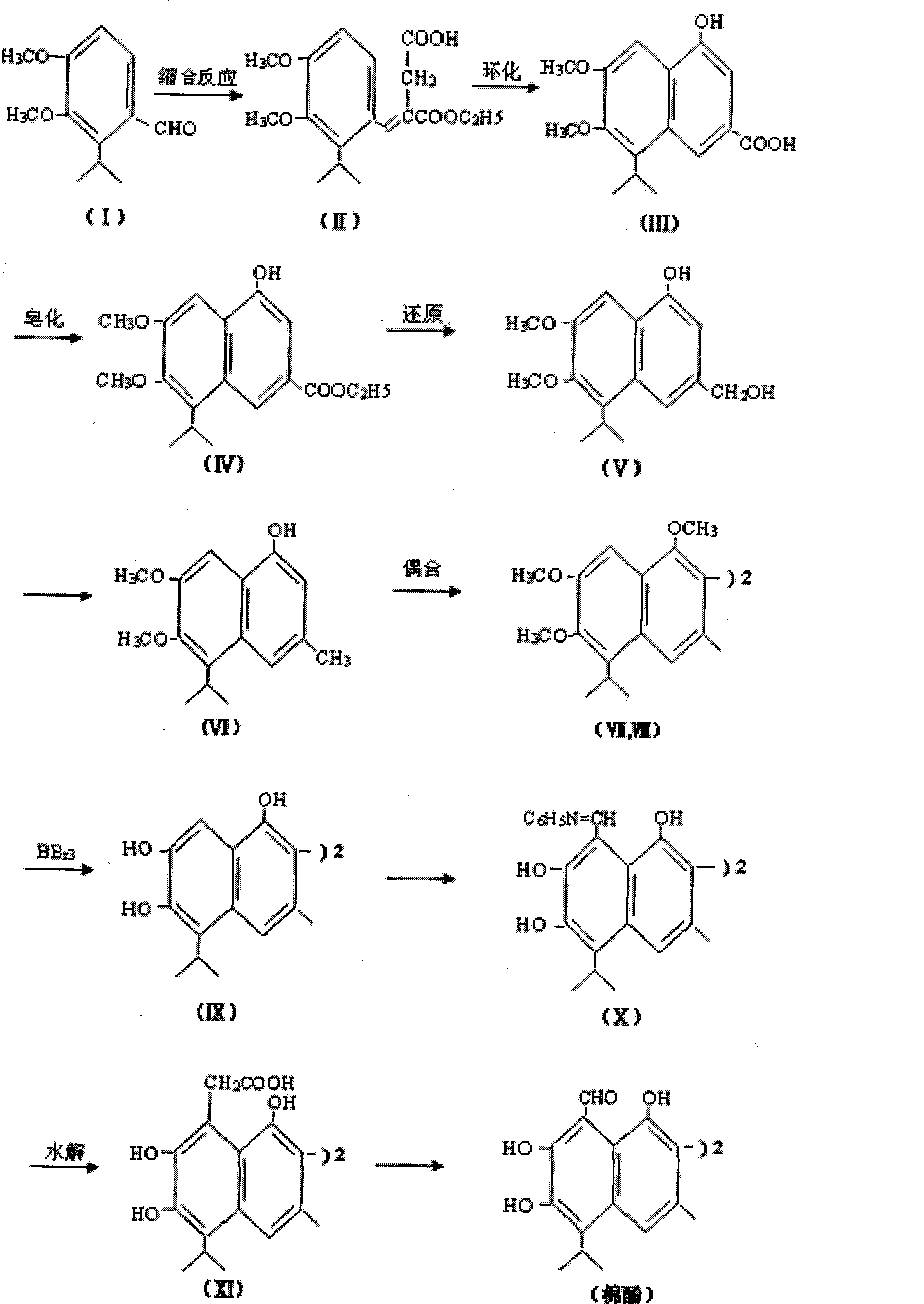
[0032] A biomimetic synthesis method of a sterile agent, mainly comprising the steps of:
[0033] I→II, condensation reaction
[0034] 3.8g N aOH was dissolved in 30 mL of anhydrous benzene to make caustic benzene solution, 27 g of diethyl succinate and 11 g of 2-isopropyl-3,4-dimethoxy-benzaldehyde were dissolved in 50 mL of benzene, and then, Add the above mixed solution under constant stirring of the alkaline benzene solution. After the mixture was heated at 50 °C for 1 h, 0.75 mL of ethanol was added. After cooling, an appropriate amount of water was added and extracted with ether. The aqueous phase was acidified and extracted with ether. The two ether extracts were combined and extracted with 250 mL of 5% aqueous sodium carbonate. The aqueous phase was acidified with dilute hydrochloric acid to give an oily product in the ether phase, which was dried over anhydrous sodium sulfate. If diethyl ether is evaporated, 14.2g of oily semi-ether product (II) can be obtained ...
Embodiment 2
[0054] A biomimetic synthesis method of a sterile agent, mainly comprising the steps of:
[0055] I→II, condensation reaction
[0056] 3.8g N a OH was dissolved in 30mL of anhydrous benzene to make a caustic benzene solution. 27 g of diethyl succinate and 11 g of 2-isopropyl-3,4-dimethoxy-benzaldehyde were dissolved in 50 mL of benzene. Then, the above mixed solution was added under constant stirring of the alkaline benzene solution. After the mixture was heated at 45 °C for 1.5 h, 0.75 mL of ethanol was added. After cooling, an appropriate amount of water was added and extracted with ether. The aqueous phase was acidified and extracted with ether. The two ether extracts were combined and extracted with 250 mL of 6% aqueous sodium carbonate. The aqueous phase was acidified with dilute hydrochloric acid to give an oily product in the ether phase, which was dried over anhydrous sodium sulfate. If diethyl ether is evaporated, 14.2g of oily semi-ether product (II) can be ob...
Embodiment 3
[0076] A biomimetic synthesis method of a sterile agent, mainly comprising the steps of:
[0077] I→II, condensation reaction
[0078] 3.8g N a OH was dissolved in 30mL of anhydrous benzene to make a caustic benzene solution. 27 g of diethyl succinate and 11 g of 2-isopropyl-3,4-dimethoxy-benzaldehyde were dissolved in 50 mL of benzene. Then, the above mixed solution was added under constant stirring of the alkaline benzene solution. After the mixture was heated at 55 °C for 1 h, 0.75 mL of ethanol was added. After cooling, an appropriate amount of water was added and extracted with ether. The aqueous phase was acidified and extracted with ether. The two ether extracts were combined and extracted with 250 mL of 5% aqueous sodium carbonate. The aqueous phase was acidified with dilute hydrochloric acid to give an oily product in the ether phase, which was dried over anhydrous sodium sulfate. If diethyl ether is evaporated, 14.2g of oily semi-ether product (II) can be obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com