Method for preparing glucuronic acid and lactone thereof by heterogenous catalytic oxidation
A technology of glucuronic acid and heterogeneous catalysis, which is applied in the field of catalyzing the oxidation of megluside to prepare glucuronic acid and its lactone, achieving the effects of saving resources, overcoming the production process, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The preparation method of the catalyst carrier used in the present invention is the citric acid complex method, promptly weighs the metal salt of the required metal component and citric acid according to the composition ratio of the carrier (the total molar ratio of citric acid and the metal ion used is 3:1) , add water to dissolve, mix, evaporate, and roast to obtain; the preparation method of the catalyst is the precipitation method, that is, the carrier obtained above is soaked in the active component solution, and the precipitant NaOH solution or ammonia water is added dropwise to deposit the active component on the carrier. , and then filtered, washed, dried and calcined to obtain the required catalyst. The preparation method of the above-mentioned carrier and catalyst can be carried out with reference to [Acta Catalytica Sinica 2002, 23(5): 413-416].
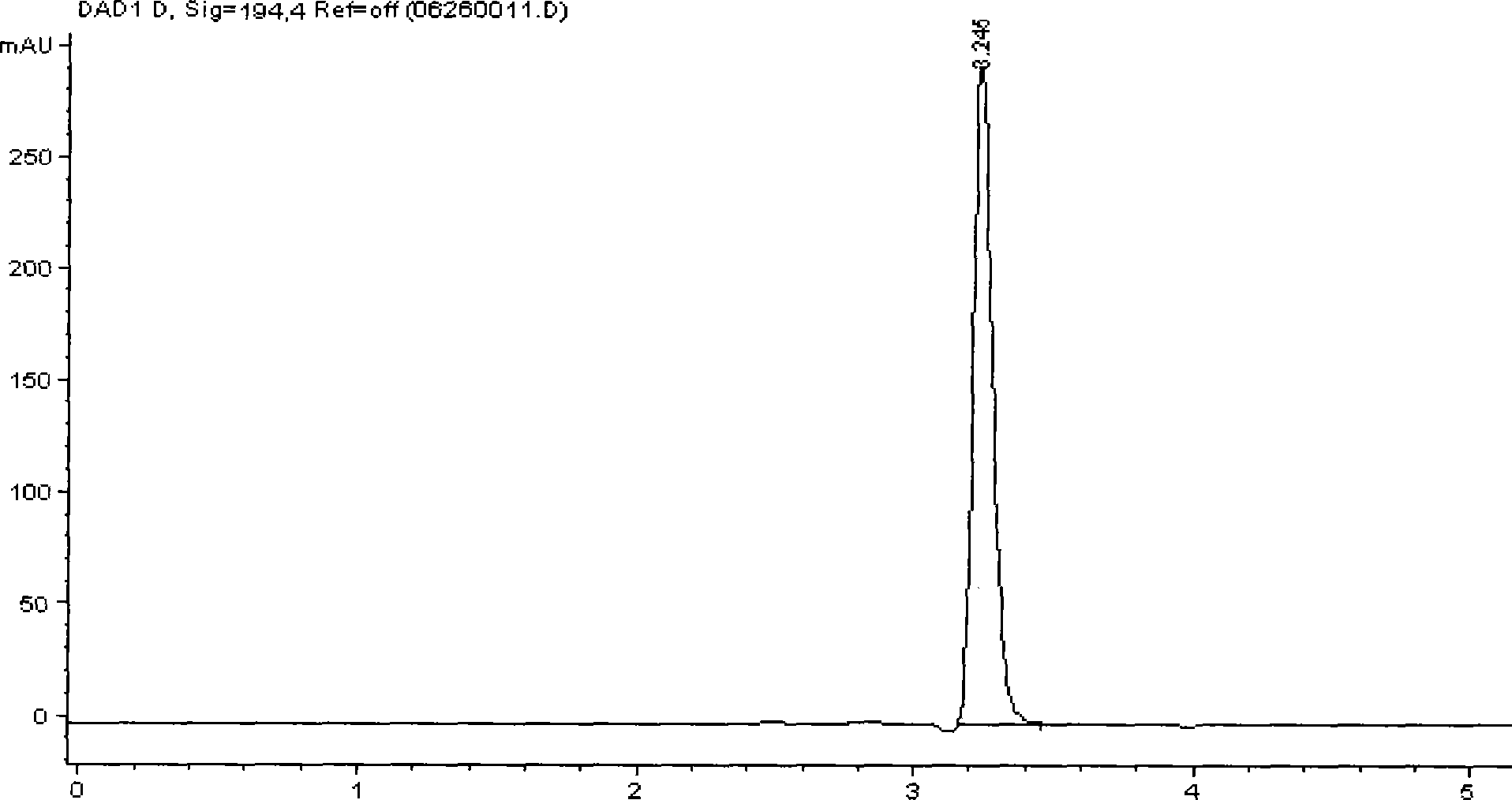
[0018] Weigh 6.0g of methyl glucoside (99.5%, industrial grade) into a reaction flask, add 50mL of distilled wate...
Embodiment 1
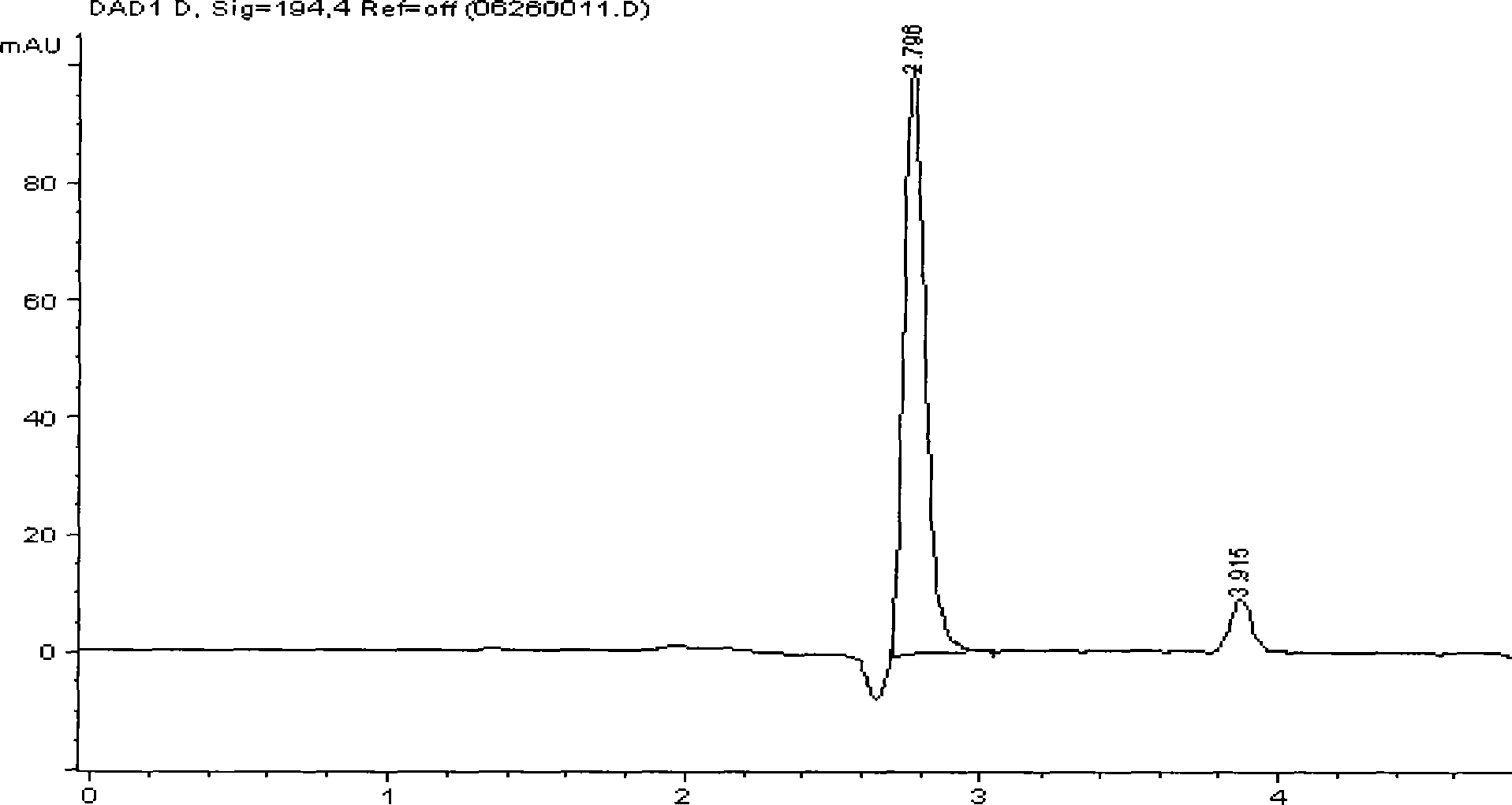
[0021] Weigh 6.0g of methyl glucoside into a three-necked flask, add 50mL of distilled water, dissolve and add 1.0g of Pd / La 0.5 Pb 0.5 MnO 3 Catalyst (the catalyst can be prepared according to the literature [Acta Catalytica Sinica 2002, 23(5): 413-416]), mechanically stirred and introduced with air. The reaction conditions are normal pressure and the temperature is 75°C. The pH value of the reaction mixture is maintained at 8-10 by dropping 1mol / L NaOH solution, and the pH value of the reaction solution is monitored with a pHs-25 digital display pH meter. After 4 hours Stop the reaction, filter the reaction mixture, adjust the pH value of the filtrate to 1-2 with dilute hydrochloric acid, and carry out hydrolysis reaction at 90°C for 3 hours. Leave standstill to cool down, take a sample and analyze the content of glucuronic acid and lactone with high performance liquid chromatography, glucuronic acid (retention time 2.809min) content reaches 29.5%, glucuronolactone (retent...
Embodiment 2
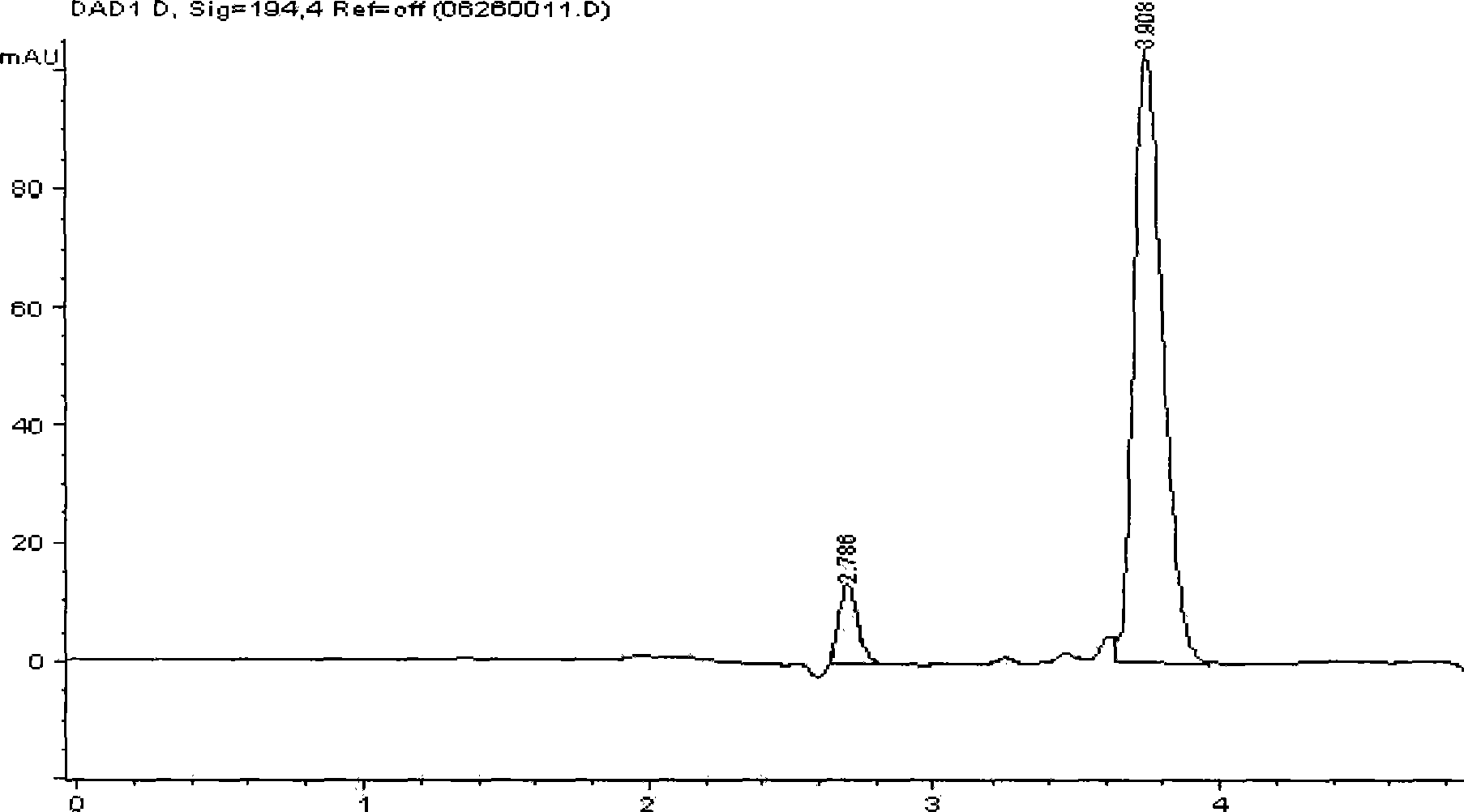
[0023] Take 6.0g methyl glucoside (99.5%, technical grade) in reaction bottle, add 50mL distilled water, add 1.0g Pd / La after dissolving 0.5 Pb 0.5 mn 0.9 sn 0.1 o 3 Catalyst (the catalyst can be prepared according to the literature [Acta Catalytica Sinica 2002, 23(5): 413-416]), mechanically stirred and introduced with air. Raise the temperature to 70°C, add 1mol / L NaOH solution dropwise to maintain the pH of the reaction mixture at 8-10, monitor the pH of the reaction solution with a pHS-25 digital display pH meter, and stop the reaction after about 5 hours. After filtering, the filtrate was adjusted to pH 1-3 with dilute hydrochloric acid, and hydrolyzed at 90°C for 3 hours. The content of glucuronic acid in the reaction process was monitored by the m-hydroxybiphenyl method. Leave it to cool, and take a sample to analyze the content of glucuronic acid and lactone by high performance liquid chromatography. The reaction result is that the content of glucuronic acid is 32....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com