Fluorocarbon nano medicine-carrying system and preparation method thereof
A technology of fluorocarbons and nano-drug loading, which is applied in the field of chemistry and can solve problems such as no drug-targeted treatment system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Precisely weigh 100mg of dexamethasone acetate powder or 20ml of dexamethasone sodium phosphate solution, add 5ml of gadopentetate meglumine, 0.6g of cholesterol, 1.2g of lecithin, 20mg of biotinylated phosphatidylethanolamine, and dissolve in about 30ml of three Chloromethane, fully oscillate and dissolve in a fume hood, add to a rotary evaporator to form a drug film, then add 20ml of perfluorocarbon solution and 1ml of safflower oil, add deionized water to make the volume 100ml, and ultrasonically shake in the cell disruptor Become microemulsion, put in the high pressure homogenizer then, low pressure (700 Pa) circulates 3 times, high pressure (1500 Pa) circulates 5 times, promptly obtains the present invention.
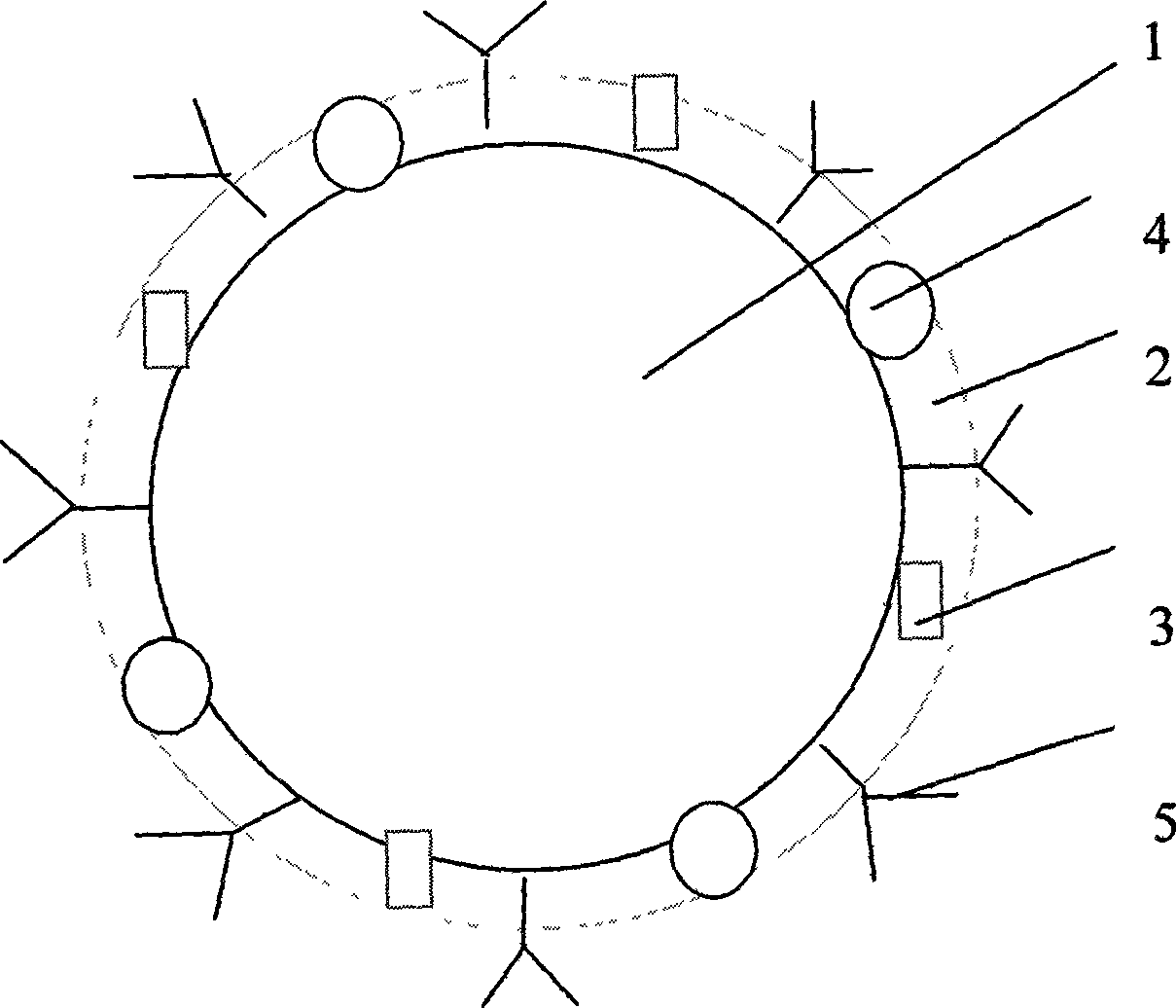
[0032] figure 1 It is a structural schematic diagram of the present invention, 1 is perfluorocarbon, 2 is lipid layer, 3 is contrast agent, 4 is glucocorticoid anti-inflammatory drug, 5 is biotinylated phosphatidylethanolamine.
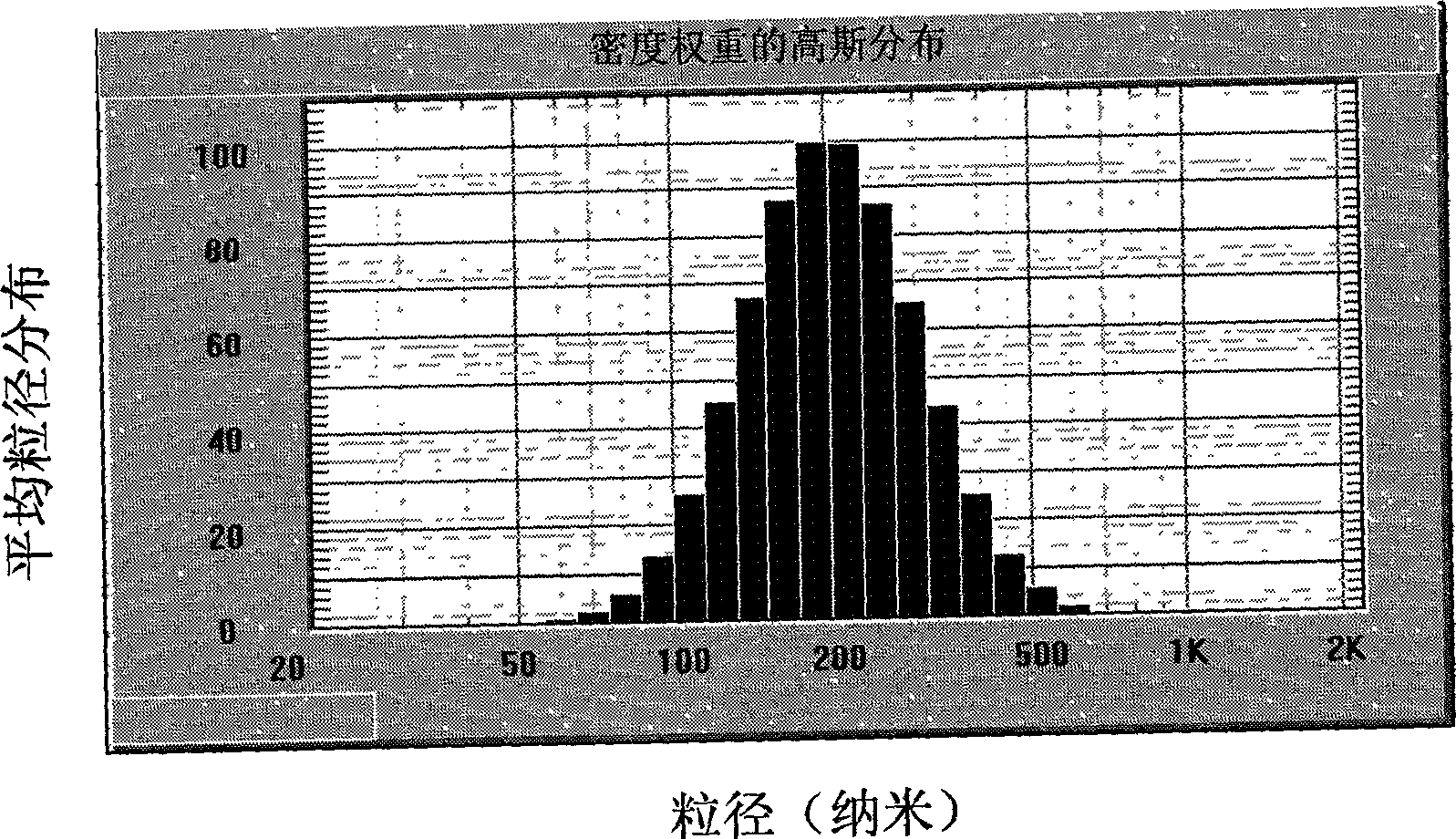
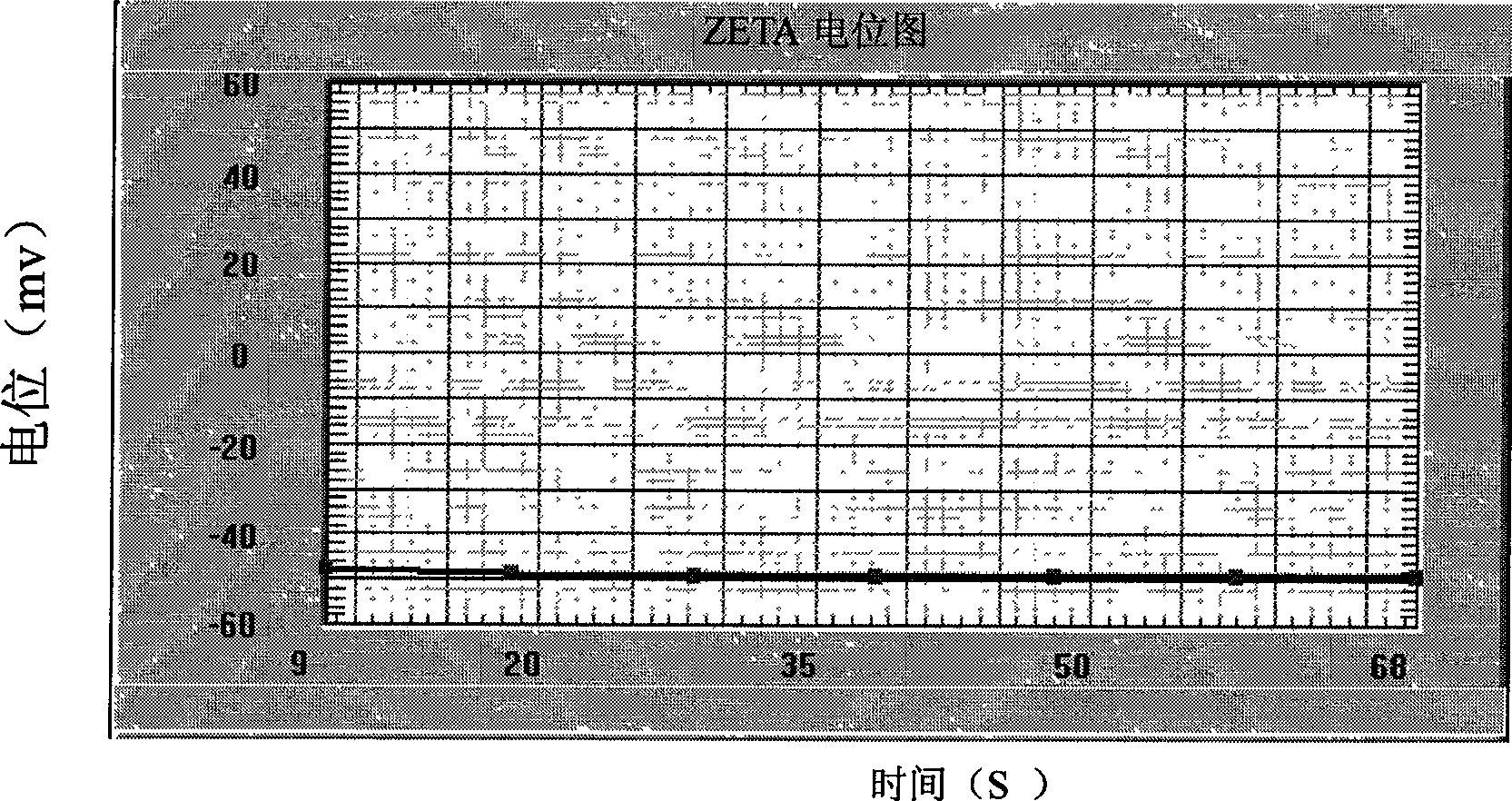
[0033] After the nano-high ...
Embodiment 2
[0035] 1. Cell inhibition test of perfluorocarbon drug-loaded nanoparticles
[0036] Take the 4th generation of SMC cultured cells, put them into a centrifuge tube after trypsinization, and centrifuge at 1000rpm for 5min; discard the supernatant, add 10% fetal bovine serum culture medium, adjust the cell density to 10000 / ml; take a 96-well plate, Inoculate 100ul of cell suspension (n=5) into the wells, the outer circle is PBS control, incubate in a 5% CO2 incubator at 37°C for 1 day; drain the culture solution, add 100ul of serum-free culture medium / well, incubate in a 5% CO2 incubator at 37°C 1d; Aspirate the culture medium, add 10% FBS DMEM containing 100ul dexamethasone (concentration 10ug / ml), perfluorocarbon drug-loaded nanometer (5%, v / v), PFC (1.5%, v / v); 37 Incubate in a 5% CO2 incubator at ℃, take out the culture plate after 3 days, add cck~8 10ul to each well and incubate for 3 hours; detect the absorbance value at 450nm wavelength with a microplate reader; Analysis...
Embodiment 4
[0049] In the in vitro release system, nanoparticles loaded with dexamethasone acetate have good sustained release. Precisely measure 5ml of nanoemulsion loaded with dexamethasone acetate (n=3) and put it into the semi-permeable membrane, and clamp it with locks at both ends. The semi-permeable membrane was placed in 200ml of human albumin saline with a concentration of 0.5mg / ml, the constant temperature shaker was set at 20°C, 60rpm, the solution was extracted and replenished regularly, and the extracted solution samples were frozen at -20°C. Specimen processing: Take 0.5ml of the specimen solution, add 0.5ml of methanol, centrifuge (10000rpm, 10min) after full shaking, and perform HPLC detection on the supernatant, the chromatographic conditions are C18 column (diamonsil), detection wavelength 240nm, mobile phase: methanol / water, 74: 26. The velocity of the mobile phase is 1ml / min, and the drug retention time is 10.41min.
[0050] The concentration linearity results of dexa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com