Process for producing 3,3',5,5'-tetramethyl-4,4'-di(2-trifluoromethyl-4-nitrophenoxy)biphenyl
A technology of nitrotrifluoromethylbenzene and nitrophenoxy is applied in the field of preparation of aromatic fluorine-containing biphenyls, can solve problems such as unfavorable environmental protection, increase three wastes, reduce production costs, etc., and achieves convenient source of raw materials, Effects with few types of use and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
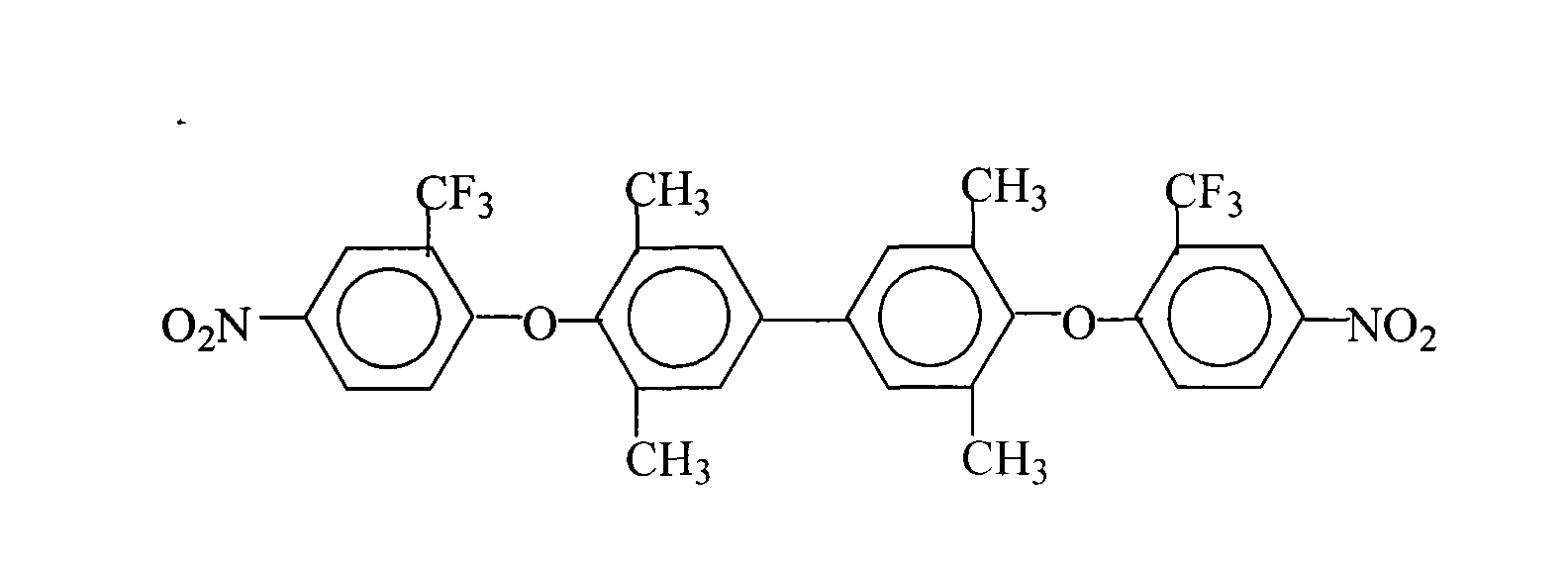
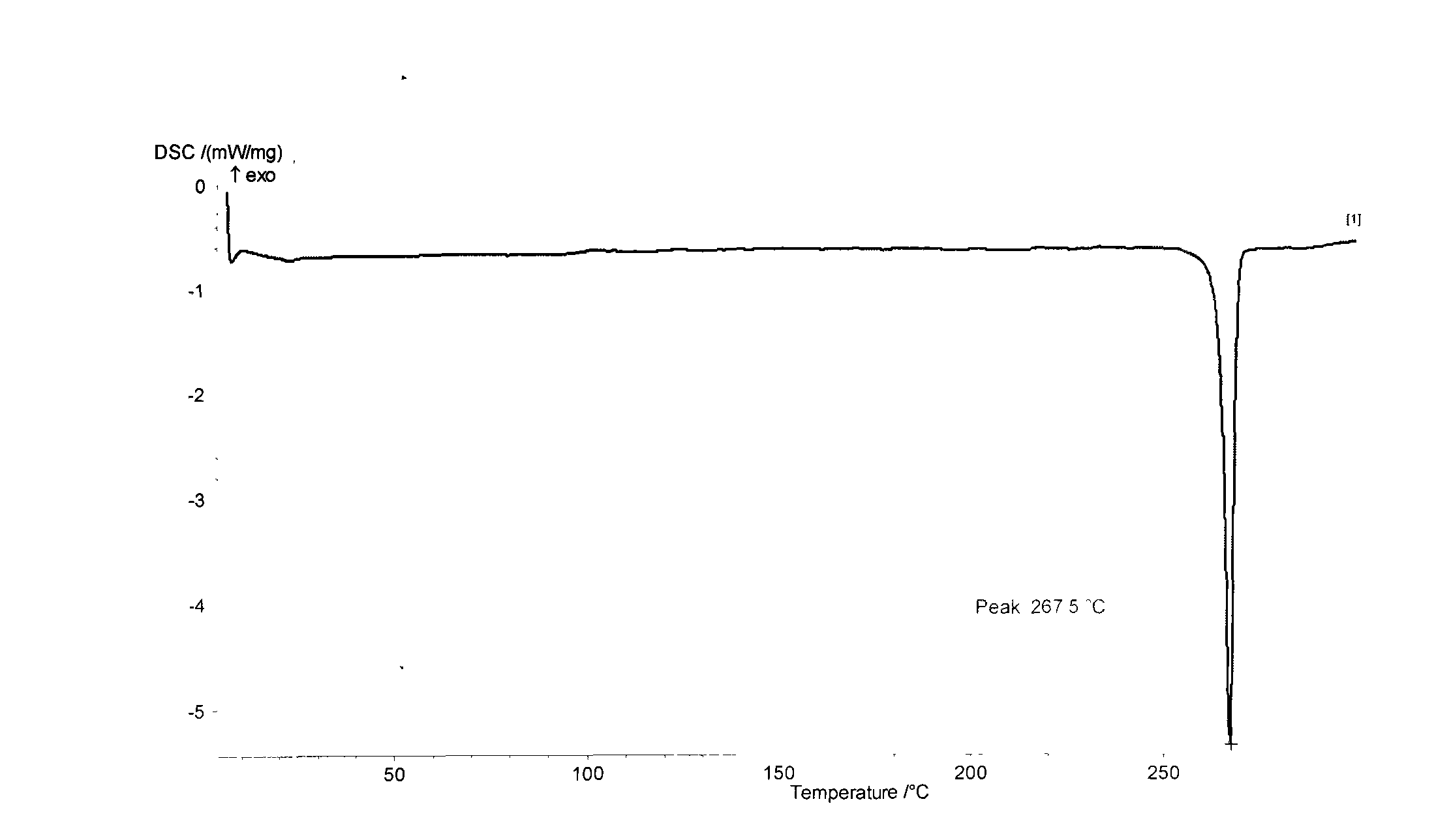
[0033] 24.2 grams (0.10 moles) of 3,3',5,5'-tetramethyl-4,4'-dihydroxybiphenyl (3355TMBP), 47.4 grams (0.21 moles) of 2-chloro-5-nitrotrifluoro A mixed solvent of methylbenzene (CNB-TF), 41.4 grams (0.30 moles) of potassium carbonate, 800 milliliters of N, N-dimethylformamide and 400 milliliters of toluene was put into a reaction kettle, stirred, and heated to 100° C.-120 ℃, reflux water separation reaction for 10 hours, continue to heat up, and react at 150°C for 1 hour, filter while hot, remove the filter residue, concentrate the mother liquor, recover the solvent for recycling, cool and stand still, and precipitate milky yellow solid crystalline product, filter, Washed 2 to 3 times with pure water, dried to obtain 59.0 grams of 3,3',5,5'-tetramethyl-4,4'-bis(2-trifluoromethyl-4-nitrophenoxy ) biphenyl (DNTMBP-2TF) milky yellow solid product (theoretical yield is 62.1 grams), the purity is 99.8%, and the fusing point is 267.5 ℃ (DSC differential scanning calorimeter, nitroge...
Embodiment 2
[0036] 24.2 grams (0.10 moles) of 3,3',5,5'-tetramethyl-4,4'-dihydroxybiphenyl (3355TMBP), 58.6 grams (0.26 moles) of 2-chloro-5-nitrotrifluoro Toluene (CNB-TF), 11.0 grams (0.08 moles) of potassium carbonate, 200 milliliters of N-methyl-2-pyrrolidone, 3600 milliliters of N, N-dimethylacetamide and 260 milliliters of xylene mixed solvents were put into the reaction Stir in the kettle, heat to 100°C-180°C, react for 15 hours, filter while hot, remove the filter residue, concentrate the mother liquor, recover the solvent for recycling, cool and stand still, precipitate a milky yellow solid product, filter, and wash with pure water 2 to 3 times, dried to obtain 57.7 grams of 3,3',5,5'-tetramethyl-4,4'-bis(2-trifluoromethyl-4-nitrophenoxy)biphenyl (DNTMBP -2TF) Creamy yellow solid product (theoretical yield: 62.1 g) with a purity of 99.5%.
[0037] Obtain 3,3',5,5'-tetramethyl-4,4'-bis(2-trifluoromethyl-4-nitrophenoxy)biphenyl (DNTMBP-2TF) according to theoretical yield and actua...
Embodiment 3
[0039] 24.2 grams (0.10 moles) of 3,3',5,5'-tetramethyl-4,4'-dihydroxybiphenyl (3355TMBP), 45.1 grams (0.20 moles) of 2-chloro-5-nitrotrifluoro The mixed solvent of methylbenzene (CNB-TF), 10.6 grams (0.10 moles) sodium carbonate, 48.3 grams (0.35 moles) salt of wormwood, 350 milliliters of N-methyl-2-pyrrolidone and 700 milliliters of toluene is put into reaction kettle, Stir, heat to 100°C-130°C, reflux and divide water for 8 hours, continue to heat up, and react at 180°C for 0.5 hours, filter while hot, remove filter residue, concentrate mother liquor, recover solvent for recycling, cool and stand still, and precipitate milk The yellow solid product was filtered, washed 2 to 3 times with pure water, and dried to obtain 55.9 grams of 3,3',5,5'-tetramethyl-4,4'-bis(2-trifluoromethyl-4- Nitrophenoxy)biphenyl (DNTMBP-2TF) milky yellow solid product with a purity of 99.6%.
[0040] According to the amount and Theoretical yield (62.1 g) was calculated to give 3,3',5,5'-tetramet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com