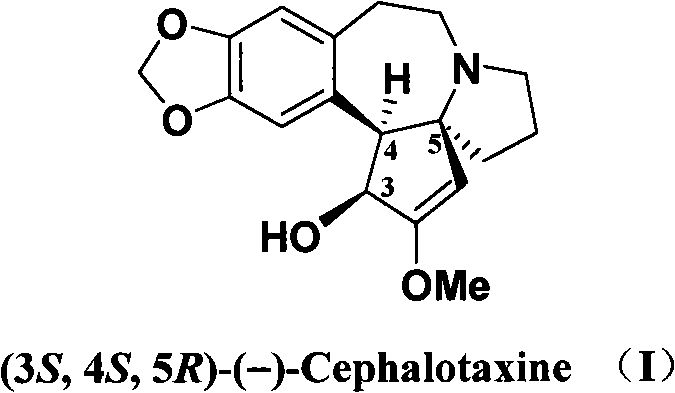
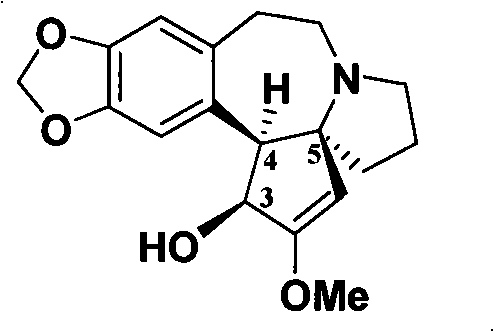
Optical pure levorotation cephalotaxine and separation purification method
A technology of L-harringtonine and harringtonine, which is applied in the field of separation and purification of optically pure L-harringtonine, and can solve problems such as lengthy synthesis lines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Extraction and purification of L-harringtonine from the total alkaloid extract of harringtonine
[0019] Get 48.4 grams of extract (prepared by the method of document J.Org.Chem.1963,28,2194, solid content 57%, containing alkaloids such as harringtonine, harringtonine and homoharringtonine), Add 300mL of water and 150mL of dichloromethane, stir, add 38mL of 2M hydrochloric acid solution dropwise, adjust the water phase to pH = 3, separate the layers, then extract the water layer with dichloromethane for 1-2 times, combine the organic phases, and recover the organic phase , to obtain trace amounts of non-alkali organic compounds and pigments.
[0020] While stirring the aqueous layer, add 42 mL of 2M aqueous sodium hydroxide solution to adjust the pH to 13, add 150 mL of diethyl ether, and stir overnight at room temperature. Add 50mL of 2M hydrochloric acid solution dropwise under stirring, adjust the water phase to pH=3, separate the liquid, then extract the ...
Embodiment 2
[0024] Example 2 Separation and purification of L-harringtonine from the residual extract after extracting harringtonine
[0025] Get 100 grams of residual extract after extracting harringtonine (solid content is about 43%, mainly harringtonine, content about 80%, excluding solvent content in the extract; document: Phytochemistry, 1972, 11, 1467) Put it into a beaker, add 500 mL of distilled water and 250 mL of dichloromethane, stir, add dropwise 70 mL of 2 M hydrochloric acid solution, adjust the water phase to pH=3, separate the layers, and then extract the water layer 1-2 with dichloromethane The second time, the organic phase is combined, and the organic phase is recovered to obtain a trace amount of non-alkali organic matter and pigment.
[0026] Add 200 mL of diethyl ether to the water layer, adjust the pH of the water phase to 13 with 75 mL of 2M sodium hydroxide solution, and stir overnight at room temperature. Add 82 mL of 2M hydrochloric acid solution dropwise under...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com