Method for preparing lansoprazole freeze-dried injection for injection
A technology of freeze-dried powder injection and lansoprazole, which is applied in the field of preparation of lansoprazole freeze-dried powder injection, can solve the problem of high insoluble particles, achieve less adverse reactions, reduce insoluble particles, improve product quality and The effect of storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 52g of lansoprazole to 200ml of isopropanol, add 17g of 33% sodium hydroxide aqueous solution to the slurry, stir at room temperature until the solution is clear, filter to remove insoluble matter, cool to 5-10°C, filter, and filter cake Wash twice with 40 ml of isopropanol at 5° C., and dry under vacuum at 50° C. to obtain 48.0 g of solid lansoprazole sodium with an HPLC purity of 99.9%.
Embodiment 2
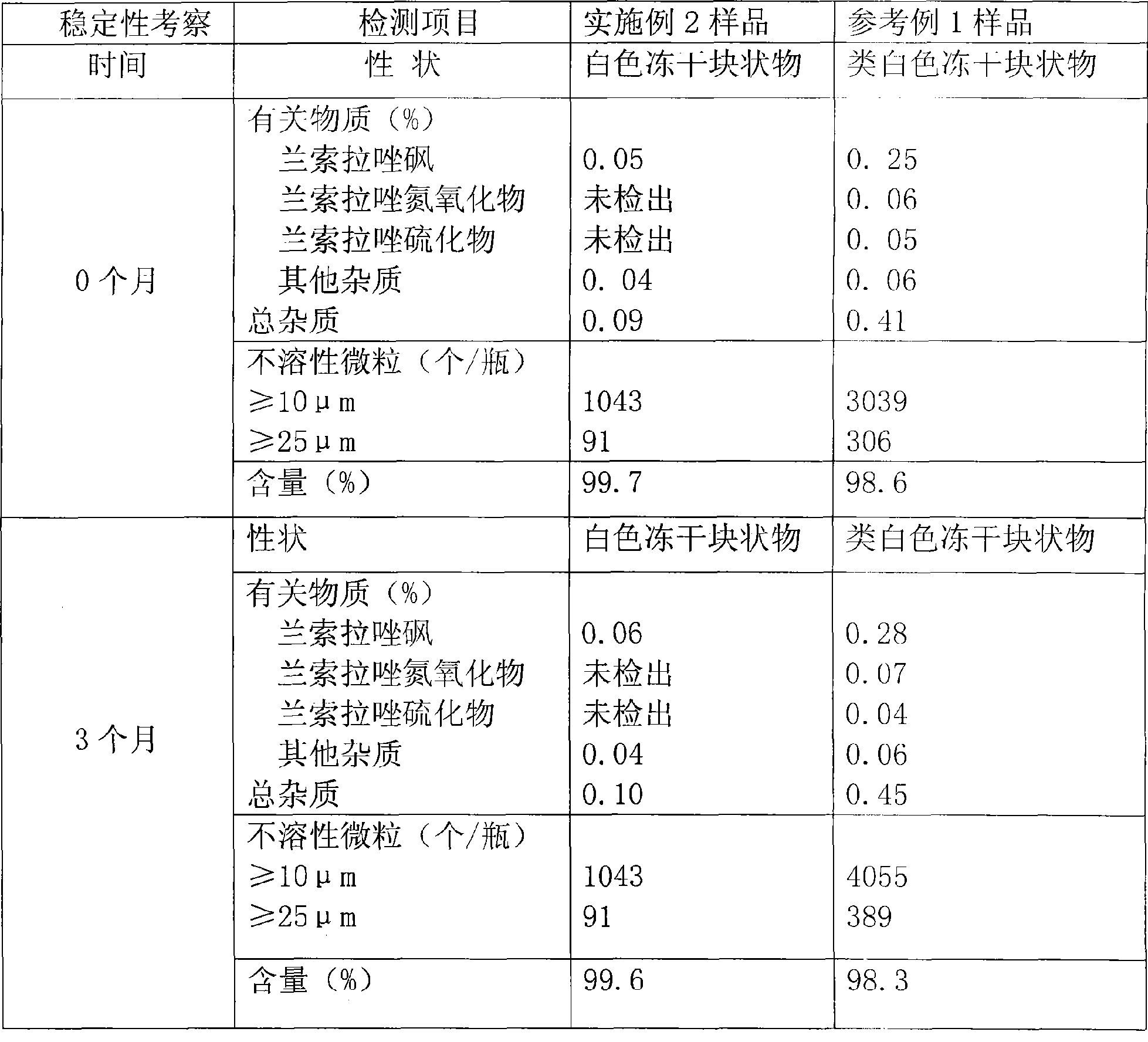
[0019] Weigh 32g of lansoprazole sodium salt, add 400ml of water for injection, stir until completely dissolved at 20-30°C to obtain solution A, weigh 10g of meglumine, add 100ml of fresh water for injection and stir until completely dissolved to obtain solution B, weigh Take 60 parts of mannitol, add 800ml of fresh water for injection, stir until completely dissolved, add 3‰ needles and stir with activated carbon for 20 minutes, decarbonize and filter to obtain solution C, first combine solution B and solution C, and then mix with solution A, Add fresh water for injection to the full volume of 2000ml. The liquid medicine is firstly filtered with a 0.45μm microporous membrane, and then sterilized and filtered with a 0.22μm microporous membrane. Sampling is used to measure the content and alkalinity pH (the pH value is controlled at 10.4-12.0 Between), heat source, and after the clarity inspection is qualified, the filtrate is half-stoppered and divided into 10ml control antibio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com