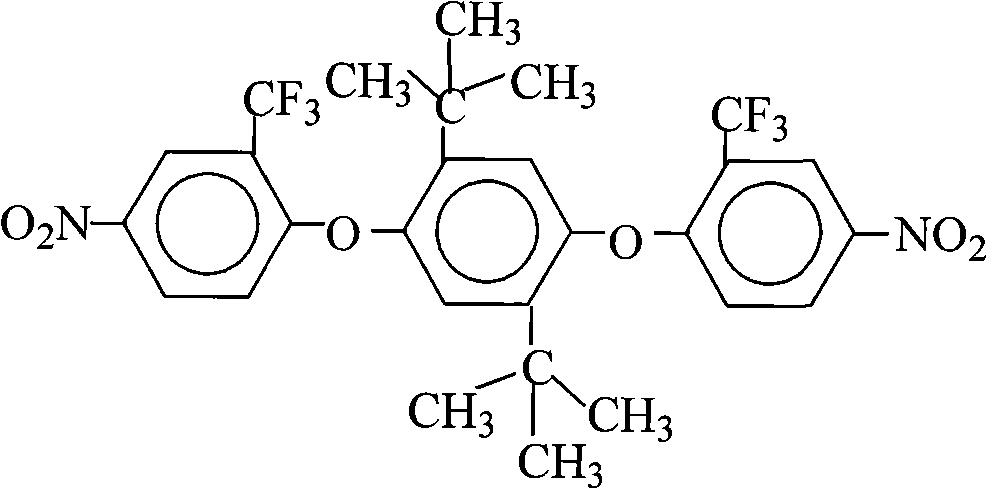
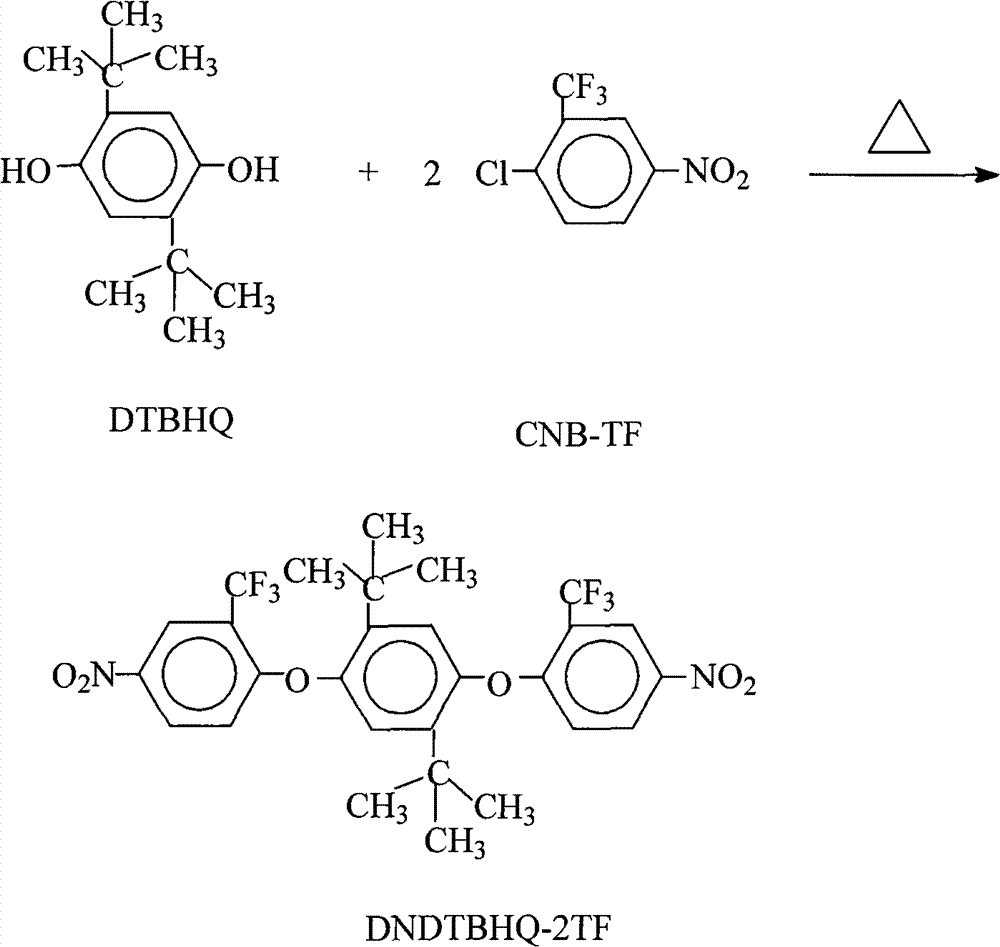
Preparation of 1,4-bis(2-trifluoromethyl-4-nitrophenoxy)-2,5-di-t-butylbenzene
A technology of nitrophenoxy and nitrotrifluoromethylbenzene is applied in the field of preparation of aromatic fluorine-containing organic compound benzene, and can solve the problem of increasing raw material cost and production cost, increasing chemical equipment and personnel cost, and increasing chemical unit operation and other problems, to achieve the effect of convenient source of raw materials, low cost and simplified process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
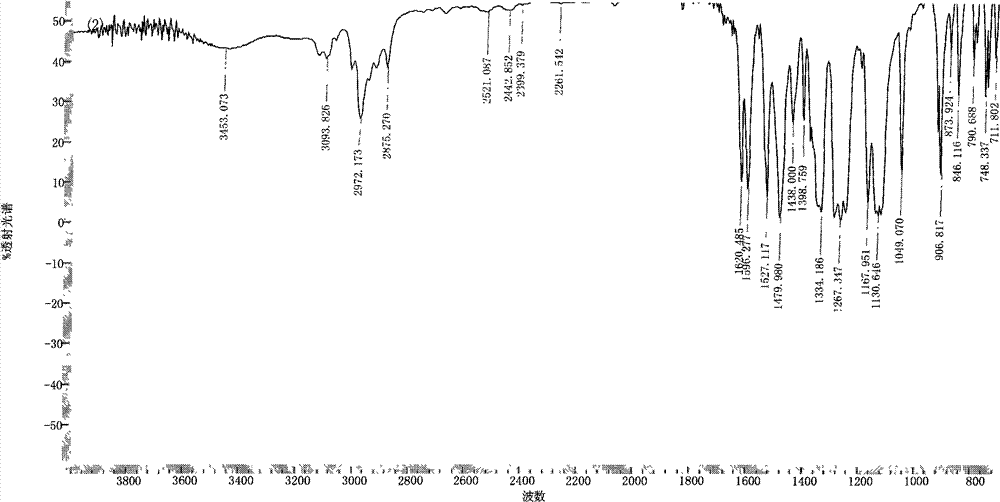
[0031] 22.2 grams (0.10 moles) of 2,5-di-tertbutyl-1,4-hydroquinone (DTBHQ), 49.6 grams (0.22 moles) of 2-chloro-5-nitrotrifluoromethylbenzene (CNB- TF), 41.4 grams (0.30 moles) of potassium carbonate, 500 milliliters of N, N-dimethylformamide and 500 milliliters of toluene mixed solvent are put into reaction kettle, stir, be heated to 120 ℃-150 ℃, reflux azeotrope After reacting with water for 15 hours, filter while it is hot, remove the filter residue, concentrate the mother liquor, recover the solvent for recycling, cool and stand still, and precipitate milky yellow solid crystalline product, filter, wash with pure water 2 to 3 times, and dry to obtain 57.0 grams of 1,4-bis(2-trifluoromethyl-4-nitrophenoxy)-2,5-di-tert-butylbenzene (DNDTBHQ-2TF) milky yellow solid product (theoretical yield is 60.0 g), the purity is 99.7%, the melting point is 265.5°C-266.8°C (WRR melting point instrument, the initial setting temperature is 258.0°C, the heating rate is 1.5°C / min), and its F...
Embodiment 2
[0033]22.2 grams (0.10 moles) of 2,5-di-tert-butyl-1,4-hydroquinone (DTBHQ), 54.1 grams (0.24 moles) of 2-chloro-5-nitrotrifluoromethylbenzene (CNB- TF), 62.1 grams (0.45 moles) of salt of wormwood, 600 milliliters of N-methyl-2-pyrrolidone, 160 milliliters of N, N-dimethylacetamide and 100 milliliters of benzene and 55 milliliters of xylene mixed solvents are put into reaction kettle , stirred, heated to 120°C-180°C, after 12 hours of azeotropic water separation reaction, filtered while hot, removed the filter residue, concentrated the mother liquor, recovered the solvent for recycling, cooled and stood still, and precipitated milky yellow solid product, filtered, and used pure Washed with water for 2-3 times and dried to obtain 55.2 g of 1,4-bis(2-trifluoromethyl-4-nitrophenoxy)-2,5-di-tert-butylbenzene (DNDTBHQ-2TF) milky yellow Solid product (theoretical yield is 60.0 grams), according to theoretical yield and actual obtain 1,4-bis(2-trifluoromethyl-4-nitrophenoxy)-2,5-di-...
Embodiment 3
[0035] 22.2 grams (0.10 moles) of 2,5-di-tertbutyl-1,4-hydroquinone (DTBHQ), 45.1 grams (0.20 moles) of 2-chloro-5-nitrotrifluoromethylbenzene (CNB- TF), 27.6 grams (0.20 moles) of potassium carbonate, 340 milliliters of N-methyl-2-pyrrolidone, 700 milliliters of o-dichlorobenzene and 1000 milliliters of toluene are put into the reaction kettle, stirred, and heated to 120°C-180°C After refluxing and azeotropic water separation for 20 hours, filter while it is hot, remove the filter residue, concentrate the mother liquor, recover the solvent for recycling, cool and stand still, and precipitate a milky yellow solid product, filter, wash 2 to 3 times with pure water, and dry to obtain 57.6 grams of 1,4-bis(2-trifluoromethyl-4-nitrophenoxy)-2,5-di-tert-butylbenzene (DNDTBHQ-2TF) milky yellow solid product, the purity is 99.7%, according to actual The amount and theoretical yield (60.0 g) of 1,4-bis(2-trifluoromethyl-4-nitrophenoxy)-2,5-di-tert-butylbenzene (DNDTBHQ-2TF) obtained w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com