Synthesis of taurine
A synthesis method and technology of taurine, applied in the preparation of sulfonic acid, organic chemistry and other directions, can solve the problems of high energy consumption, high labor intensity and high production cost, and achieve the effects of good product quality, low production cost and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
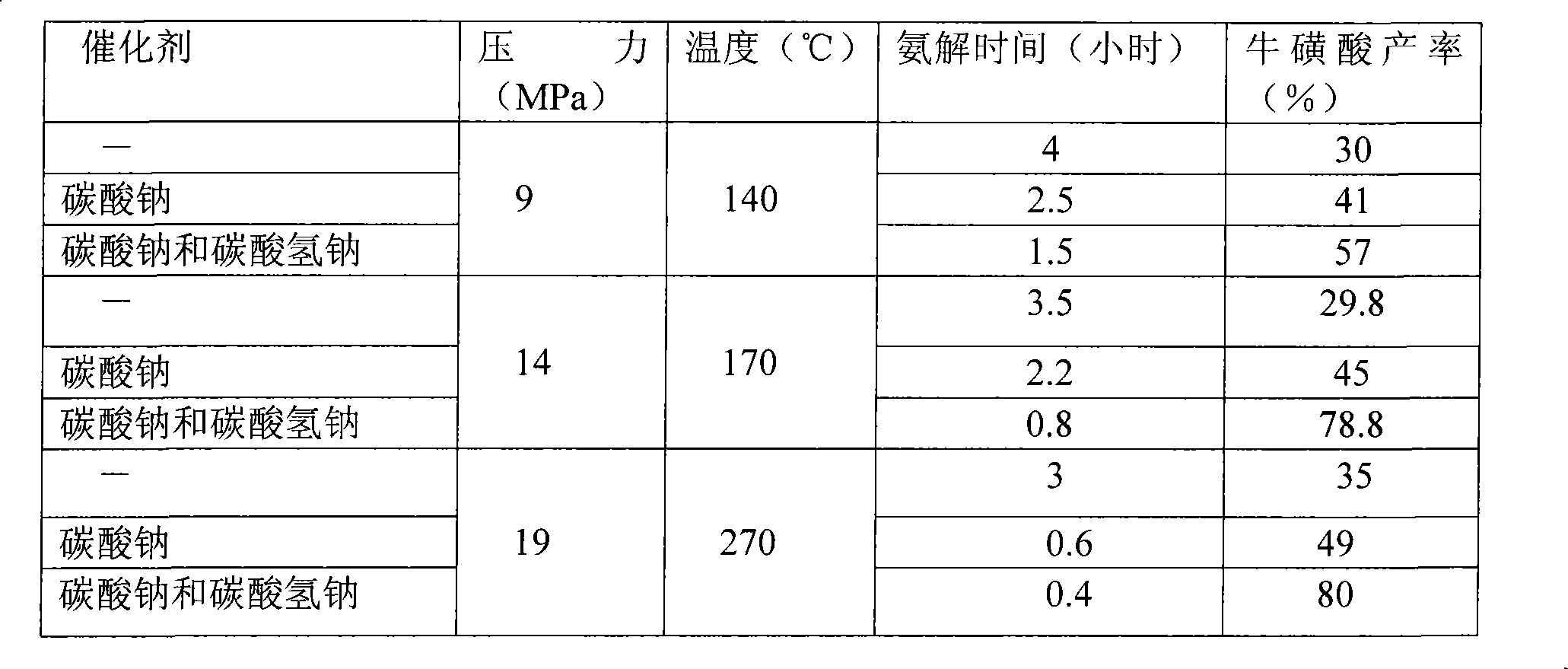
[0017] The preparation of sodium isethionate: feed ethylene oxide gas in 24wt% sodium bisulfite solution (the molar ratio of ethylene oxide and sodium bisulfite is 1:1.2) at 0.1MPa, pH Value 6.5-7.5 and react at 80°C for 1.2 hours to obtain a mixture containing sodium isethionate (99% conversion).
[0018] The preparation of sodium taurate: in the presence of catalyst sodium carbonate and sodium bicarbonate (the mol ratio of sodium carbonate and sodium bicarbonate is 3:1), in the mixture containing sodium isethionate, add liquid ammonia (reaction The concentration of ammonia in the liquid is 20% (mass percent), reacted at 15MPa and 160° C. for 45 minutes to obtain sodium taurate; wherein, the consumption of the catalyst was 1% of the quality of sodium isethionate.
[0019] Preparation of taurine: neutralize sulfuric acid and sodium taurine at a molar ratio of 1:2 at 30-70°C, and crystallize at 10-50°C to obtain the product taurine with a yield of 73.4%.
[0020] 1 H NMR (DMS...
Embodiment 2
[0022] Preparation of sodium isethionate: react 1:1.1 molar ratio of ethylene oxide and sodium bisulfite at 0.07MPa, pH 6.5-7.5 and 75°C for 1.5 hours to prepare sodium isethionate .
[0023] The preparation of sodium taurate: in the presence of catalyst sodium carbonate and sodium bicarbonate (the mol ratio of sodium carbonate and sodium bicarbonate is 5:1), sodium isethionate and liquid ammonia (the concentration of ammonia in the reaction solution 25%, mass percent) reacted at 21MPa and 280° C. for 25 minutes to prepare sodium taurate; wherein, the amount of catalyst was 5% of the mass of sodium isethionate.
[0024] Preparation of taurine: Neutralize sulfuric acid and sodium taurine at 30-70°C at a molar ratio of 1:2.1 to obtain the product taurine with a yield of 81.5%. 1 H NMR (DMSO-d 6 , TMS, 90MHz) δ 2.73(m, 2H), 3.05(m, 2H), 7.64(br, 3H); IR(cm -1 ): 3209.87, 3050.12, 2969.21 (-NH 2 ); 1179.11, 1222.15, 1511.09, 1585.06, 1616.32 (-SO 3 H); 13 C-NMR: δ 550.3 (C1)...
Embodiment 3
[0026] Preparation of sodium isethionate: react 1:1 molar ratio of ethylene oxide and sodium bisulfite at 0.08MPa, pH 6.5—7.5 and 85°C for 1.1 hours to prepare sodium isethionate .
[0027] The preparation of sodium taurate: in the presence of catalyst sodium carbonate and sodium bicarbonate (the mol ratio of sodium carbonate and sodium bicarbonate is 4:1), sodium isethionate and liquid ammonia (the concentration of ammonia in the reaction solution 30%, mass percent) was reacted at 17MPa and 190° C. for 30 minutes to prepare sodium taurate; wherein, the amount of catalyst was 3% of the mass of sodium isethionate.
[0028] Preparation of taurine: Neutralize sulfuric acid and sodium taurine at 30-70°C at a molar ratio of 1:2.2 to obtain the product taurine with a yield of 78.6%. 1 H NMR (DMSO-d 6 , TMS, 90MHz) δ 2.73 (m, 2H), 3.06 (m, 2H), 7.66 (br, 3H); IR (cm -1 ): 3210.17, 3049.88, 2968.98 (-NH 2 ); 1179.15, 1221.87, 1511.25, 1584.86, 1616.18 (-SO 3 H); 13 C-NMR: δ 550....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com