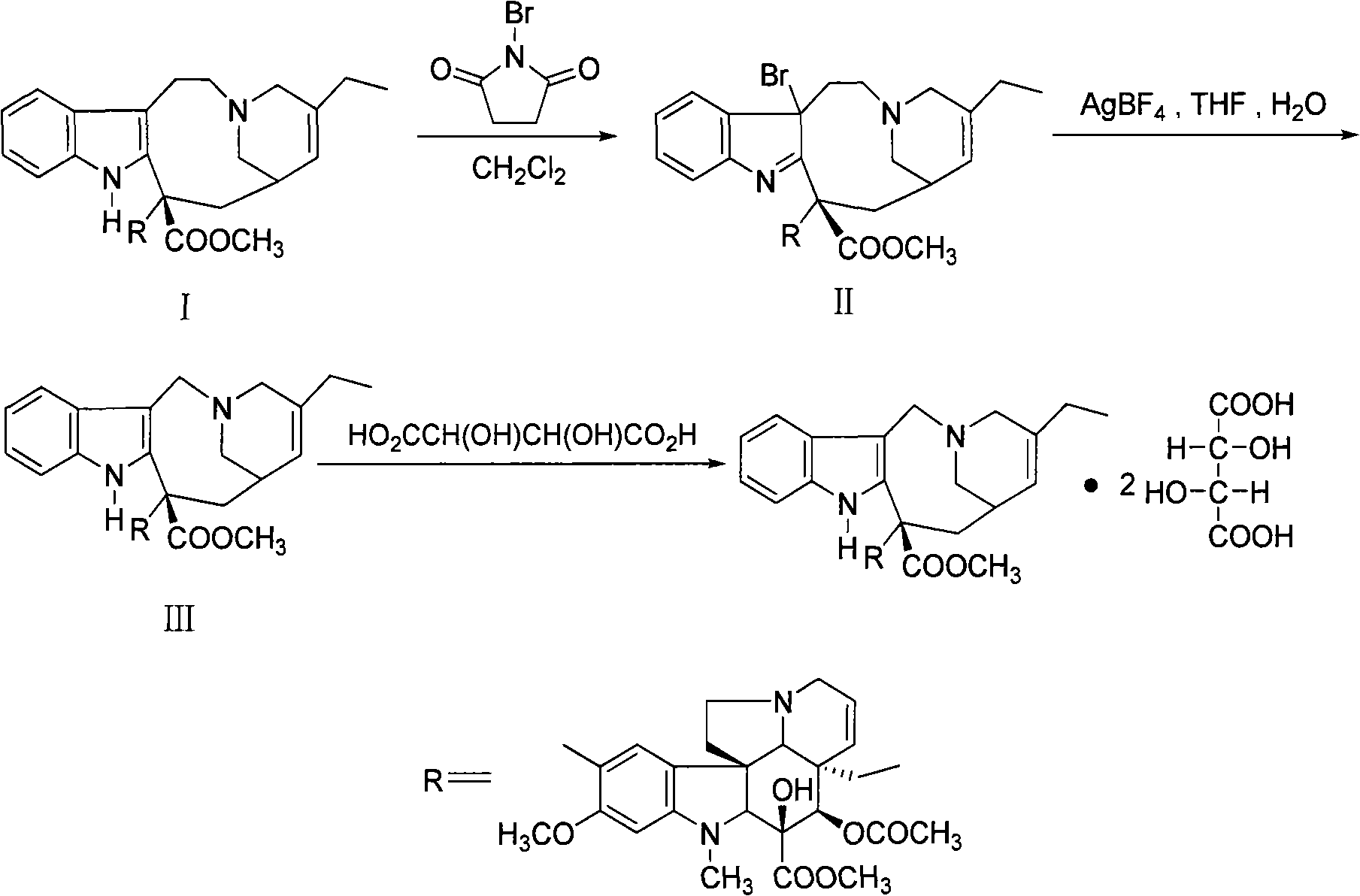
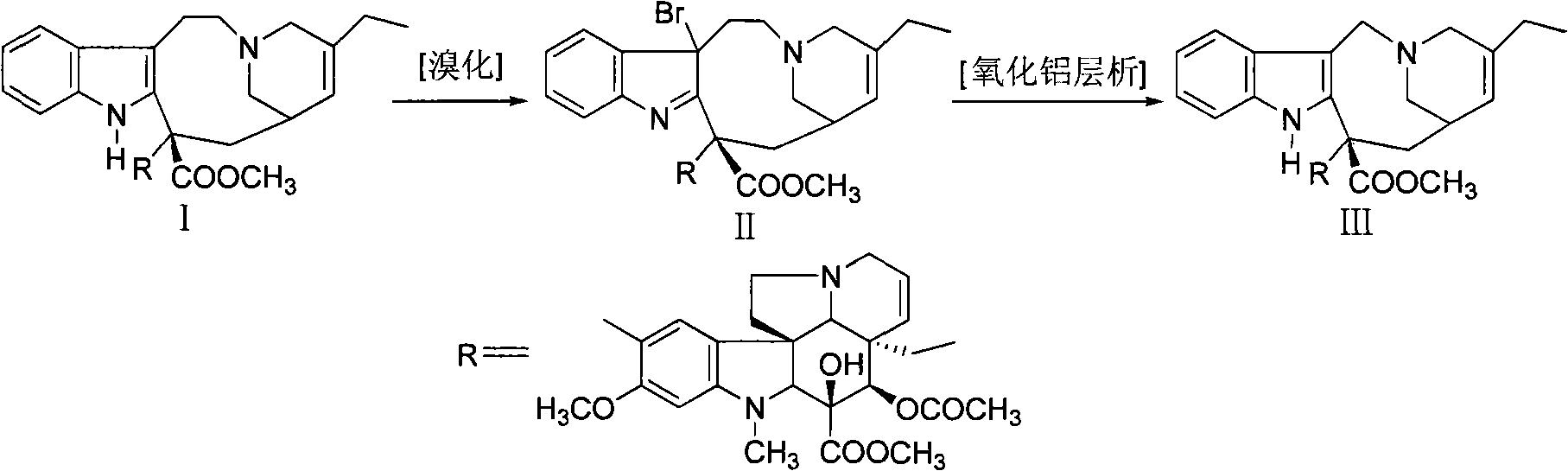
Preparation method for vinorelbine
A technology of vinorelbine and dehydrated vinblastine, which is applied in the field of pharmaceutical preparation, can solve the problems of high price of silver tetrafluoroborate reagent, long process flow, complicated operation and the like, and achieves broad industrial application prospect, shortened process flow and preparation technology. optimized effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Under nitrogen protection, 10g of dehydrated vinblastine (purity 90.3% HPLC) was dissolved in 100ml of dichloromethane, cooled to -65°C, slowly added 2ml of trifluoroacetic acid, 50ml of dichloromethane and 2.3g of N-bromobutylene The solution composed of imide was added and reacted below -70°C for 2 hours (thin-layer chromatography showed that the starting material disappeared). Add 60ml of saturated sodium bicarbonate solution, stir evenly, let stand to separate layers, extract the aqueous layer twice with dichloromethane (25ml×2), combine the organic phases, wash with 30ml of water, and concentrate under reduced pressure to obtain bromide concentrate .
[0021] Put the bromide concentrated solution on the alumina column (the amount of alumina is 200g), use dichloromethane-methanol gradient elution, thin-layer chromatography (chloroform: methanol = 95: 5) monitoring, collect vinorelbine-containing fractions, after merging Concentrate under reduced pressure to obtain ...
Embodiment 2
[0023] 48.5g dehydrated vinblastine purity (91.36%), 485ml dichloromethane were added in the reaction flask, stirred and dissolved under nitrogen protection, cooled to -65°C, slowly added 5ml trifluoroacetic acid, 300ml dichloromethane and 11g N -A solution composed of bromophthalimide was added and reacted below -70°C for 2 hours (thin-layer chromatography showed that the raw materials disappeared). Add 500ml of saturated sodium bicarbonate solution, stir evenly, let stand to separate layers, extract the aqueous layer twice with dichloromethane (100ml×2), combine the organic phases, wash with 200ml of water, and concentrate under reduced pressure to obtain bromide concentrate .
[0024] Put the concentrated bromide solution on an alumina column (amount of alumina is 1500g), elute with dichloromethane-methanol gradient, monitor by thin-layer chromatography, collect vinorelbine-containing fractions, combine and concentrate under reduced pressure to obtain 42.6g vinorelbine Bin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com