Preparation method of glycin chelated iron nano liposome dispersion liquid
A technology of glycine chelated iron and nano-liposomes, which is applied in food preparation, application, food science, etc., can solve the problem of low bioavailability of glycine chelated iron, lower bioavailability, and no glycine chelated iron nano-lipid The report of plastid preparation and other issues, to achieve the effect of improving bioavailability, enhancing stability, and good iron supplementation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
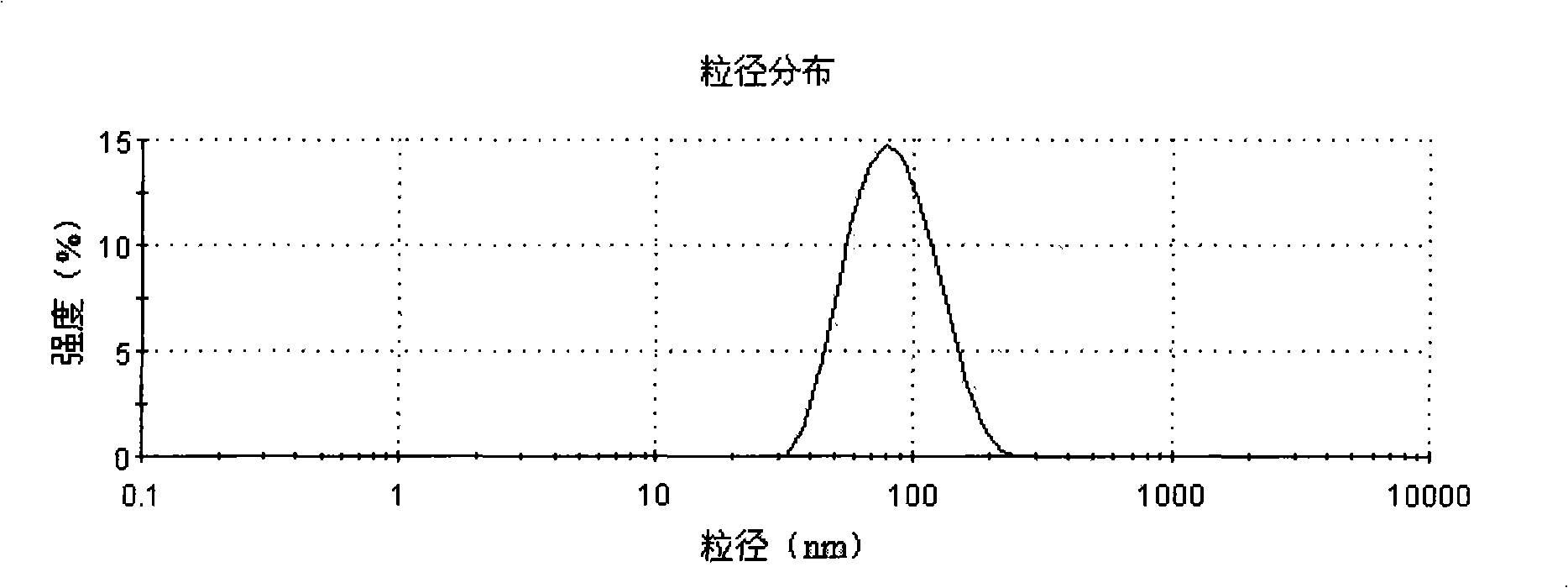
Image
Examples
Embodiment 1
[0017] Weigh 200 mg of lecithin and 50 mg of cholesterol, dissolve in 9 mL of anhydrous ether (organic phase); weigh 20 mg of glycine chelated iron and dissolve in 3 mL of citric acid-disodium hydrogen phosphate buffer (pH 6.8) (water phase). Gained organic phase and aqueous phase were mixed, and after 6 minutes of probe-type ultrasonic treatment (intensity 80%, 1 second on, 1 second off) to form a stable and uniform water-in-oil emulsion, transferred to a 250mL rotary evaporation flask, 35 Rotate the ether in a water bath at ℃ to remove the ether. A gel will form in 5 to 10 minutes. As the rotary evaporation continues, the gel collapses. Keep the rotary evaporation for 15 to 30 minutes to remove the residual ether. Add 12 mL of citric acid-disodium hydrogen phosphate buffer solution (pH 6.8) containing 200 mg Tween 80 to the evaporation flask, and hydrate with rotation in a water bath at 35° C. for 20 minutes. The volume of the hydration solution is 75 times the mass of lecit...
Embodiment 2
[0019] Weigh 300 mg of lecithin and 30 mg of cholesterol, dissolve in 15 mL of anhydrous ether (organic phase); weigh 30 mg of glycine chelated iron and dissolve in 5 mL of citric acid-disodium hydrogen phosphate buffer (pH 6.8) (water phase). Gained organic phase and aqueous phase were mixed, and after 10 minutes of probe-type ultrasonic treatment (intensity 60%, 1 second on, 1 second off) to form a stable and uniform water-in-oil emulsion, transferred to a 250mL rotary evaporator flask, 40 Rotate the ether in a water bath at ℃ to remove the ether. A gel will form in 5 to 10 minutes. As the rotary evaporation continues, the gel collapses. Keep the rotary evaporation for 15 to 30 minutes to remove the residual ether. Add 20 mL of citric acid-disodium hydrogen phosphate buffer solution (pH 6.8) containing 300 mg Tween 80 to the evaporating flask, and hydrate with rotation in a water bath at 40° C. for 15 minutes. The volume of the hydration solution is 83 times the mass of leci...
Embodiment 3
[0021] Weigh 200mg of lecithin and 50mg of cholesterol, dissolve in 10mL of anhydrous ether, transfer to a 250mL rotary evaporating flask, remove the ether by rotary evaporation in a 35°C water bath, and form a uniform layer of lipid film on the bottle wall. After the film is formed, Add 9 mL of anhydrous ether to the evaporating flask to redissolve to obtain an organic phase. Weigh 20 mg of iron glycine chelate and dissolve in 3 mL of citric acid-disodium hydrogen phosphate buffer (pH 6.8) (water phase). Gained organic phase and aqueous phase were mixed, and after 6 minutes of probe-type ultrasonic treatment (intensity 90%, 1 second on, 1 second off) to form a stable and uniform water-in-oil emulsion, transferred to a 250mL rotary evaporator flask, 40 Rotate the ether in a water bath at ℃ to remove the ether. A gel will form in 5 to 10 minutes. As the rotary evaporation continues, the gel collapses. Keep the rotary evaporation for 15 to 30 minutes to remove the residual ether...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com