Oxime ester photoinitiator and preparation method thereof
A technology of photoinitiator and oxime ester, applied in the field of oxime ester photoinitiator and preparation thereof, can solve problems such as difficulty in curing colored resists, and achieve the effects of improving initiation activity, low production cost and increasing compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
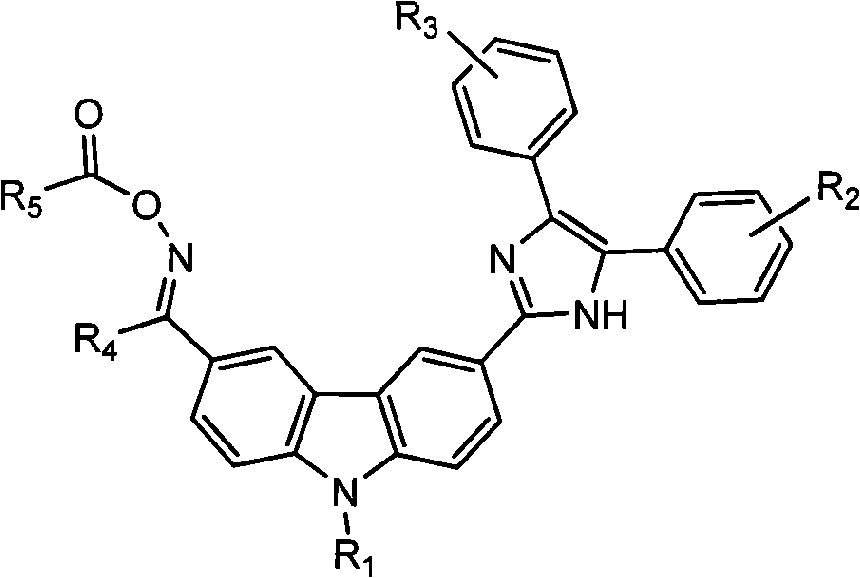
[0030] According to the photoinitiator of present embodiment, chemical name is N-ethylcarbazole-3-imidazole (2,3-diphenyl)-6-isobutylketone oxime ethyl ester, and its structural formula is as follows:
[0031]
[0032] It is prepared by following steps carried out in sequence:
[0033] (1), the synthesis of N-ethylcarbazole-3-isobutylone-6-formaldehyde
[0034]
[0035]Add 44.6g (0.2mol) of N-ethylcarbazole-3-carboxaldehyde and 250ml of anhydrous dichloroethane to a 500ml four-neck flask equipped with electric stirring, reflux condenser, dropping funnel and tail gas absorption device, and stir After dissolving (yellow), slowly add 58.1g (0.435mol) of anhydrous aluminum trichloride, the heat release is obvious, control the temperature not to exceed 40°C, keep stirring at 20-40°C for 10-15 minutes, then stir and cool to 5 ℃ or so. Slowly add the dichloroethane solution of isobutyryl chloride [composed of 23.8g isobutyryl chloride (0.223mol) and 20ml dichloroethane] dropw...
Embodiment 2
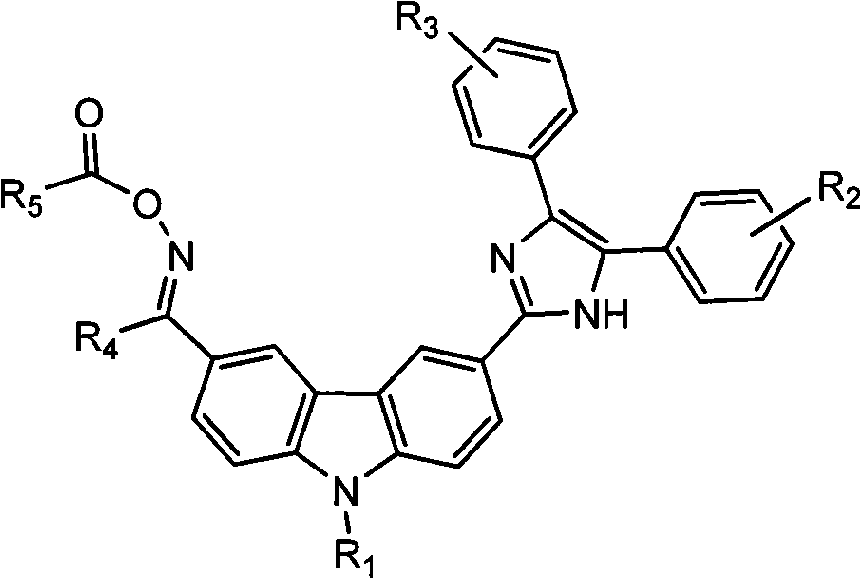
[0046] According to the photoinitiator of the present embodiment, the chemical name is N-ethylcarbazole-3-imidazole (2,3-diphenyl)-6-isobutanone oxime benzyl ester, and its structural formula is as follows:
[0047]
[0048] This photoinitiator is prepared through the following steps:
[0049] (1), synthesize N-ethylcarbazole-3-imidazole (2,3-diphenyl)-6-isobutylone oxime according to the method of embodiment 1;
[0050] (2), generate N-ethylcarbazole-3-imidazole (2,3-diphenyl)-6-isobutanone oxime and benzoyl chloride reaction by N-ethylcarbazole-3-imidazole (2, 3-Diphenyl)-6-isobutanone oxime benzyl ester.
[0051]
[0052] Add 2.5g (0.005mol) of N-ethylcarbazole-3-imidazole (2,3-diphenyl)-6-isobutylketone oxime (content 96.4%) (synthetic with example 1), THF 50g, after stirring and dissolving, be cooled to 0-5 ℃, add triethylamine 0.9g (0.009mol), keep 0-5 ℃, dropwise the THF solution of benzoyl chloride ( 1.2g of benzoyl chloride (0.0085mol), 5g of tetrahydrofuran)...
Embodiment 3
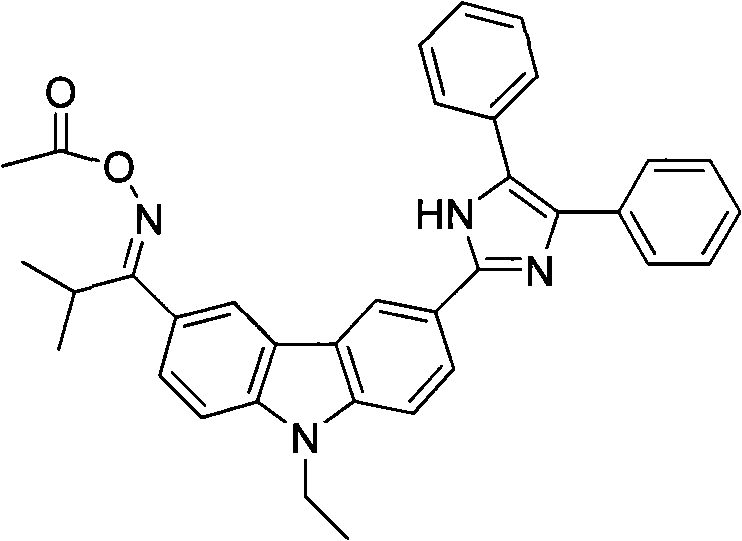
[0054] According to the photoinitiator of the present embodiment, its chemical structural formula is as follows:
[0055]
[0056] This photoinitiator can be prepared according to the method identical with embodiment 1 and 2, and its 1 H-NMR (ppm) is: 13.067, 7.692, 7.891, 7.711, 8.776, 7.053, 7.062, 7.552, 7.071, 7.063, 7.541, 7.533, 7.884, 7.653, 8.218, 7.561, 7.588, 8.235, 8.6676, 7. 3.832, 3.805, 4.534, 1.767, 1.305, 1.066.
[0057] mp 195-197°C, decomposition temperature 346°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com