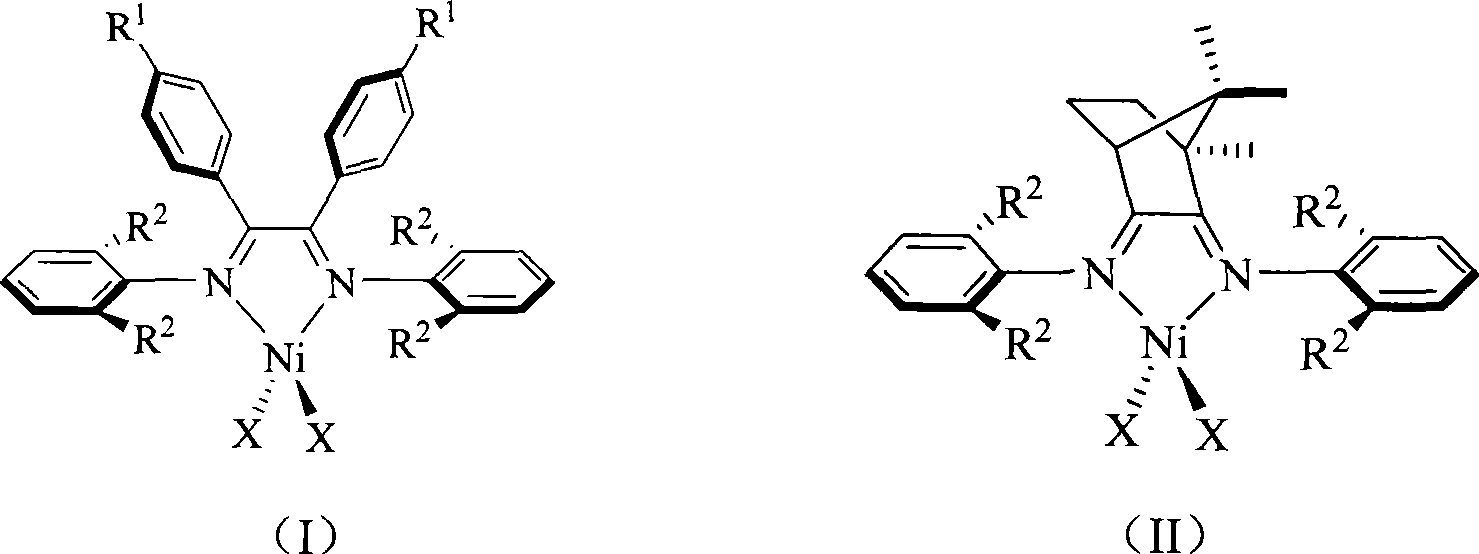
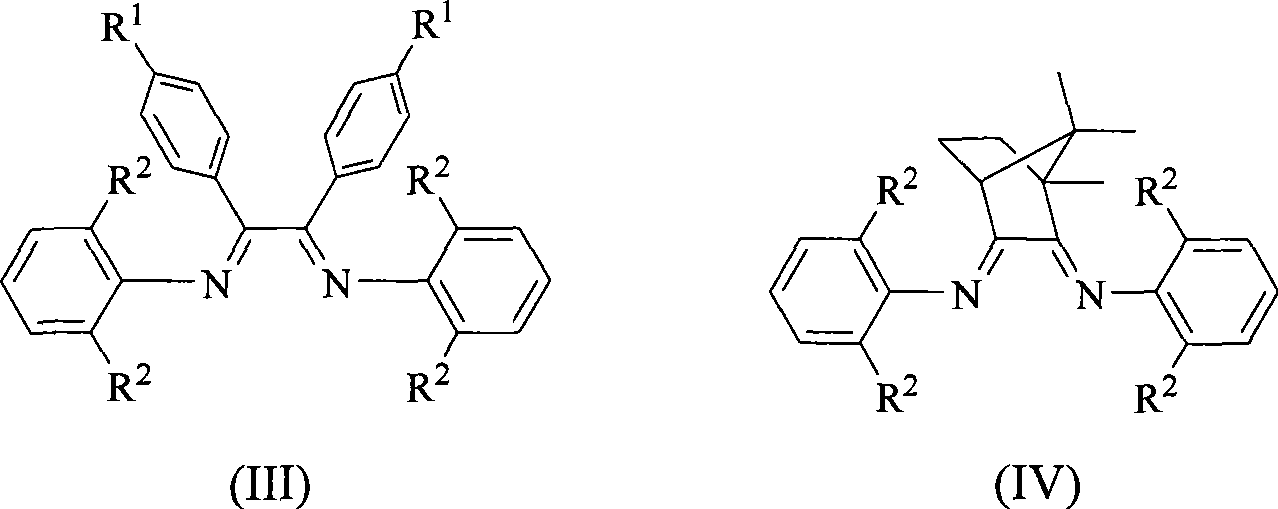
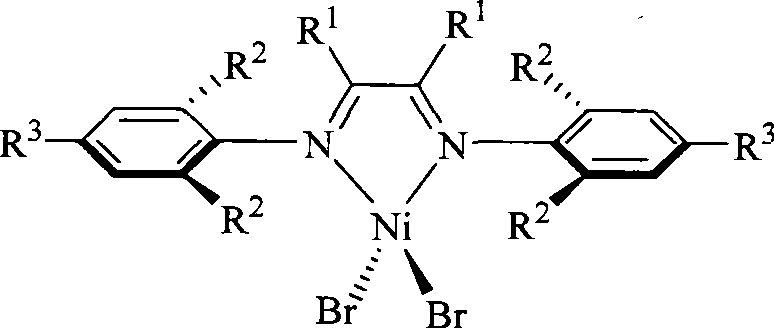
Alpha-nickel diimine compound olefin polymerization catalyst and preparation method thereof, and method for preparing branched polyethylene
A technology for olefin polymerization and diimine nickel is applied in the field of α-diimine nickel olefin polymerization catalyst and its preparation, and in the field of catalyzing ethylene polymerization to prepare branched polyethylene, which can solve the problems of decomposition and deactivation, poor thermal stability and the like, and achieves the The effect of prolonging catalytic life, low cost, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of Ligand L1a
[0044] Under nitrogen atmosphere and room temperature, 20ml of toluene, 1.45g (1.5ml, 12mmol) of 2,6-dimethylaniline, and 12ml of trimethylaluminum (1.0M, 12mmol) were successively added into a 100ml branch bottle. React at 110° C. for 2 hours, lower the reaction temperature to room temperature, and then add 1.05 g (5 mmol) of dibenzoyl. At this time, the reaction system changed from colorless to deep red, and released a lot of heat. The reaction was continued at 110° C. for 6 hours, and then the temperature was lowered to 0° C., and the reaction system was terminated with 5% sodium hydroxide ice solution. The organic phase was extracted with ethyl acetate, followed by anhydrous MgSO 4After drying, the solvent was spin-dried to obtain an orange oil. The product was separated through a silica gel column, eluent (petroleum ether / ethyl acetate=15:1). Recrystallization in ethanol gave orange crystals, yield: 69.2%. NMR test shows that the liga...
Embodiment 2
[0046] Synthesis of Ligand L2a
[0047] According to the synthesis method of ligand L1a in Example 1, 4,4'-dimethyldibenzoyl was used instead of dibenzoyl, and other operating conditions were the same. An orange oil was obtained, yield: 74.1%. NMR test shows that the ligand has isomers in the solution state, and the isomer ratio is 2.5:1. 1 H NMR (300MHz, CDCl 3 ), δ (ppm): Isomer 1: 8.12-6.82 (m, 14H, Ar-H), 2.46 (s, 12H, CH 3 at aniline phenyl), 1.79(s, 6H, CH 3 at benzil backbone). Isomer 2: 8.12-6.82(m, 14H, Ar-H), 2.22(s, 12H, CH 3 at aniline phenyl), 1.59(s, 6H, CH 3 at benzilbackbone). 13 C NMR (75MHz, CDCl 3 ), δ (ppm): Isomer 1: 164.49 (C=N), 147.60 (C-N), 140.91 (CC=N), 131.77 (C Ar -Me), 128.49, 127.30, 126.32125.25, 122.80, 21.46, 18.67. Isomer 2: 165.00 (C=N), 147.33 (C-N), 139.88 (CC=N), 132.59 (C Ar -Me), 129.12, 127.50, 126.78, 124.91, 122.93, 21.31, 18.50. Elemental analysis result C 32 h 32 N 2 : Theoretical value: C, 86.44; H, 7.25; N, 6.3...
Embodiment 3
[0049] Synthesis of Ligand L2b
[0050] According to the synthetic method of ligand L1a in Example 1, replace 2,6-dimethylaniline with 2,6-diisopropylaniline and replace dibenzoyl with 4,4'-dimethyldibenzoyl, and others operating conditions are the same. Orange crystals were obtained, yield: 48.6%.
[0051] The NMR test showed that the ligand has isomers in the solution state, and the isomer ratio is 1.9:1. 1 H NMR (300MHz, CDCl 3 ), δ (ppm): Isomer 1: 7.87-6.86 (m, 14H, Ar-H), 2.98 (m, 4H, CH (CH 3 ) 2 ), 2.29 (s, 6H, CH 3 ), 1.12(m, 24H, CH(CH 3 ) 2 ). Isomer 2: 7.87-6.86 (m, 14H, Ar-H), 3.13 (m, 4H, CH (CH 3 ) 2 ), 2.39 (s, 6H, CH 3 ), 0.96(m, 24H, CH(CH 3 ) 2 ). 13 C NMR (75MHz, CDCl 3 ), δ (ppm): Isomer 1: 166.29 (C=N), 145.74 (C-N), 140.34 (CC=N), 134.97 (C Ar -Me), 129.07, 128.59, 123.43, 122.97, 28.55, 23.65, 21.44. Isomer 2: 166.19 (C=N), 142.56 (C-N), 139.97 (CC=N), 134.76 (C Ar -Me), 129.07, 128.59, 123.43, 122.97, 27.89, 24.48, 22.63. Elemental anal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting temperature | aaaaa | aaaaa |
| Melting temperature | aaaaa | aaaaa |
| Melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com