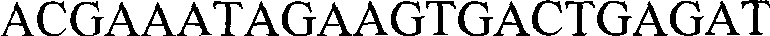Sutellaria viscidula phenyl alanine ammonialyase protein coded sequence
A technology of phenylalanine ammonia-lyase protein and coding sequence, which is applied in the field of genetic engineering, and can solve problems such as the cloning of the flavonoid pal gene of Scutellaria baicalensis, which has not been seen and has not yet been discovered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Cloning of phenylalanine ammonia-lyase from Scutellaria baicalensis
[0077] 1. Tissue separation (isolation)
[0078] Scutellaria baicalensis was purchased from Shanxi University, and the seeds were sown in the greenhouse. When the Scutellaria baicalensis seedlings grew to a height of 10 cm, DNA or RNA was prepared to be extracted.
[0079] 2. RNA isolation (RNA isolation)
[0080] Take part of the tissue, grind it with a mortar, add it to a 1.5mL EP tube filled with lysate, shake it fully, and then transfer it into a glass homogenizer. After homogenization, transfer to 1.5mL EP tube, and extract total RNA (TRIzol Reagents, GIBCO BRL, USA). The quality of total RNA was identified by formaldehyde denaturing gel electrophoresis, and then the RNA content was determined on a spectrophotometer.
[0081] 3. Cloning of Full-length cDNA
[0082] According to the nucleotide conservative sequence of the phenylalanine ammonia-lyase gene of various plants, using the principle ...
Embodiment 2
[0093] Sequence information and homology analysis of phenylalanine ammonia lyase gene from Scutellaria baicalensis
[0094] The full-length cDNA of the new phenylalanine ammonia-lyase gene sequence of the present invention is 2406bp in length, and the detailed sequence is shown in SEQID NO.3, wherein the open reading frame is located at 129-2261 nucleotides (2133 nucleotides). According to the full-length cDNA, the amino acid sequence of phenylalanine ammonia-lyase from Scutellaria baicalensis was deduced, with a total of 711 amino acid residues, a molecular weight of 77.18kDa, and an isoelectric point (PI) of 5.97. See SEQ ID NO.4 for the detailed sequence.
[0095] The full-length cDNA sequence related to the phenylalanine ammonia-lyase gene gene of Scutellaria baicalensis and its encoded protein were published in Non-redundant GenBank+EMBL+DDBJ+PDB and Non-redundant GenBankCDS translations+PDB+SwissProt+Superdate+ Nucleotide and protein homology search was carried out in t...
Embodiment 3
[0097] The protein or polypeptide of Scutellaria baicalensis phenylalanine ammonia-lyase was expressed in eukaryotic cells in Scutellaria baicalensis and the content of baicalin in transgenic plants was detected.
[0098] The construction of the expression vector containing the target gene, according to the Scutellaria baicalensis phenylalanine ammonia-lyase gene coding sequence (SEQID NO.3), design and amplify the primers for the complete coding reading frame, and introduce them on the upstream and downstream primers respectively Restriction endonuclease sites (this can be selected depending on the carrier), in order to construct expression vectors. Using the amplified product obtained in Example 1 as a template, after PCR amplification, the Scutellaria baicalensis phenylalanine ammonia-lyase gene cDNA was cloned into an intermediate vector (such as pBluescript), and further cloned into a binary expression vector (such as pBI121 and improved pCAMBIA1304), the expression vecto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com