Novel tin amino-alkoxide complexes and process for preparing thereof
一种烷氧基锡、络合物的技术,应用在氨基烷氧基锡络合物及其制备领域,达到高热稳定性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (Dimethylamino-2-methyl-2-propoxy)Sn(II)[Sn(dmamp) 2 ] preparation
[0048] SnCl at room temperature 2 (1g, 5.27mmol) and lithium bis(trimethylsilyl)amide [Li(btsa)] (1.76g, 10.54mmol) were charged into a 250mL Schlenk flask, ether (50mL) was added in the flask, and then Stir for 3 hours. The mixed solution was filtered to remove LiCl, and the solvent was removed from the filtrate under vacuum. at 10 -2 torr was fractionally distilled at 100°C to obtain Sn(btsa) 2 . Sn(btsa) 2 (1 g, 2.28 mmol) was dissolved in n-hexane. 2 equivalents of dimethylamino-2-methyl-2-propanol (0.53 g, 4.56 mmol) were slowly added thereto at room temperature, followed by stirring for 6 hours.
[0049] After removing the solvent under vacuum, at 10 -2 fractional distillation of torr at 100°C to obtain pure Sn(dmamp) 2 Compound (91%).
[0050] C 12 h 28 N 2 o 2 Sn H 2 Elemental analysis of O {calculated (measured)}: C, 39.05 (37.92); H, 8.19 (7.66); N, 7.59 (8.02)
[0051] The S...
Embodiment 2
[0054] (Dimethylamino-2-methyl-2-propoxy)Sn(II)[Sn(dmamp) 2 ] preparation
[0055] SnBr at room temperature 2 (1g, 3.59mmol) and dimethylamino-2-methyl-2-propoxysodium [Na(dmamp)] (1g, 7.18mmol) were charged into a 250mL Schlenk flask, and THF (50mL ), followed by reflux for 12 hours. The mixed solution was filtered to remove NaCl, and the solvent was removed from the filtrate under vacuum. at 10 -2 Fractional distillation of torr at 100°C afforded pure compound (93%).
[0056] C 12 h 28 N 2 o 2 Sn H 2 Elemental analysis of O {calculated (measured)}: C, 39.05 (37.92); H, 8.19 (7.66); N, 7.59 (8.02)
[0057] Therefore, it was confirmed that the same compound as that of Example 1 was produced.
Embodiment 3
[0059] (Dimethylamino-2-methyl-2-butoxy)Sn(II)[Sn(dmamb) 2 ] preparation
[0060] SnCl at room temperature 2 (1g, 5.27mmol) and lithium bis(trimethylsilyl)amide [Li(btsa)] (1.76g, 10.54mmol) were charged into a 250mL Schlenk flask, ether (50mL) was added in the flask, and then Stir for 3 hours. The mixed solution was filtered to remove LiCl, and the solvent was removed from the filtrate under vacuum. at 10 -2 torr was fractionally distilled at 100°C to obtain Sn(btsa) 2 . Sn(btsa) 2 Dissolved in n-hexane. 2 equivalents of dimethylamino-2-methyl-2-butanol (0.59 g, 4.56 mmol) were slowly added thereto at room temperature, followed by stirring for 6 hours. After removing the solvent under vacuum, at 10 -2 fractional distillation of torr at 120°C to obtain pure Sn(dmamb) 2 compound (89%).
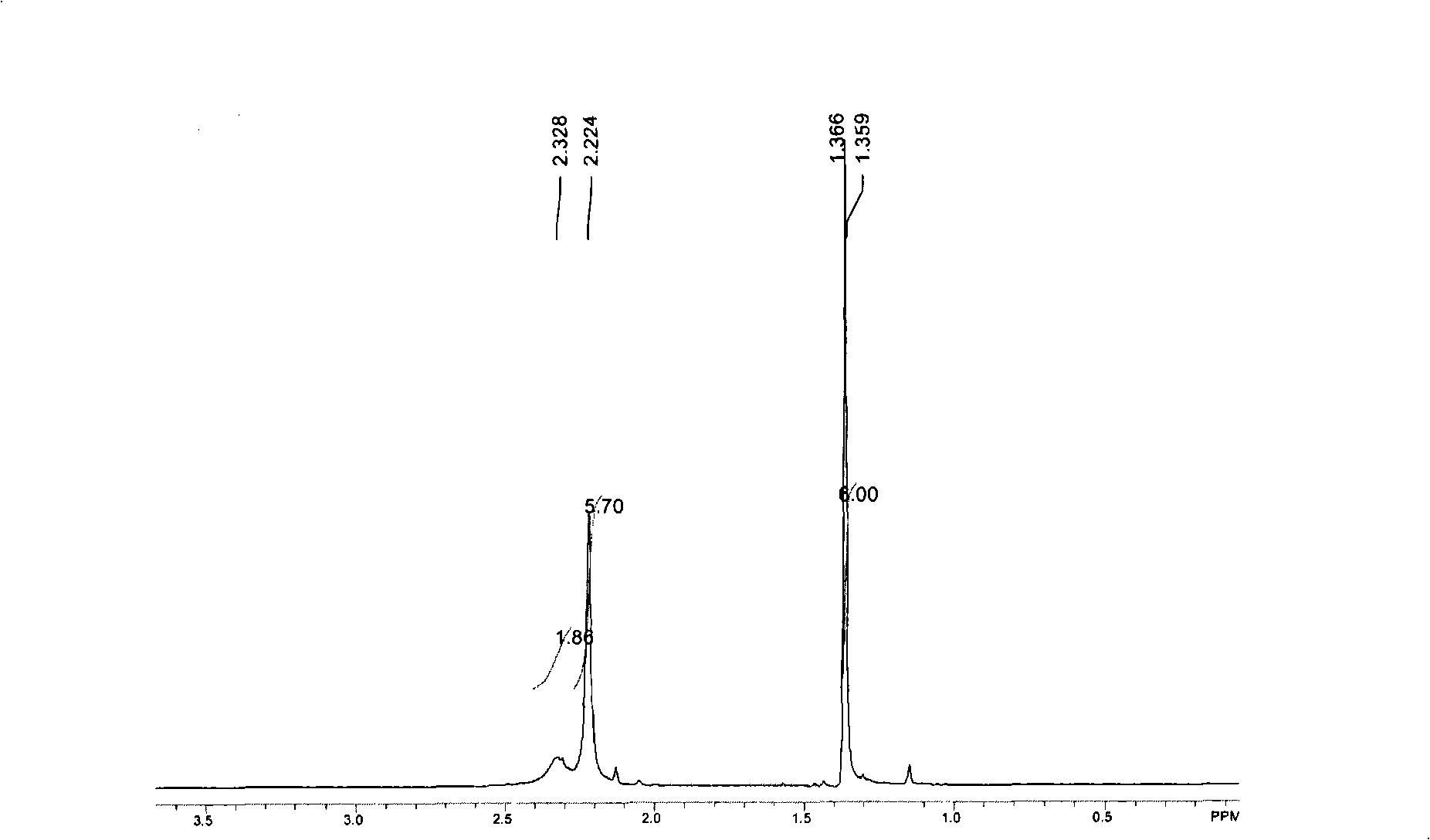
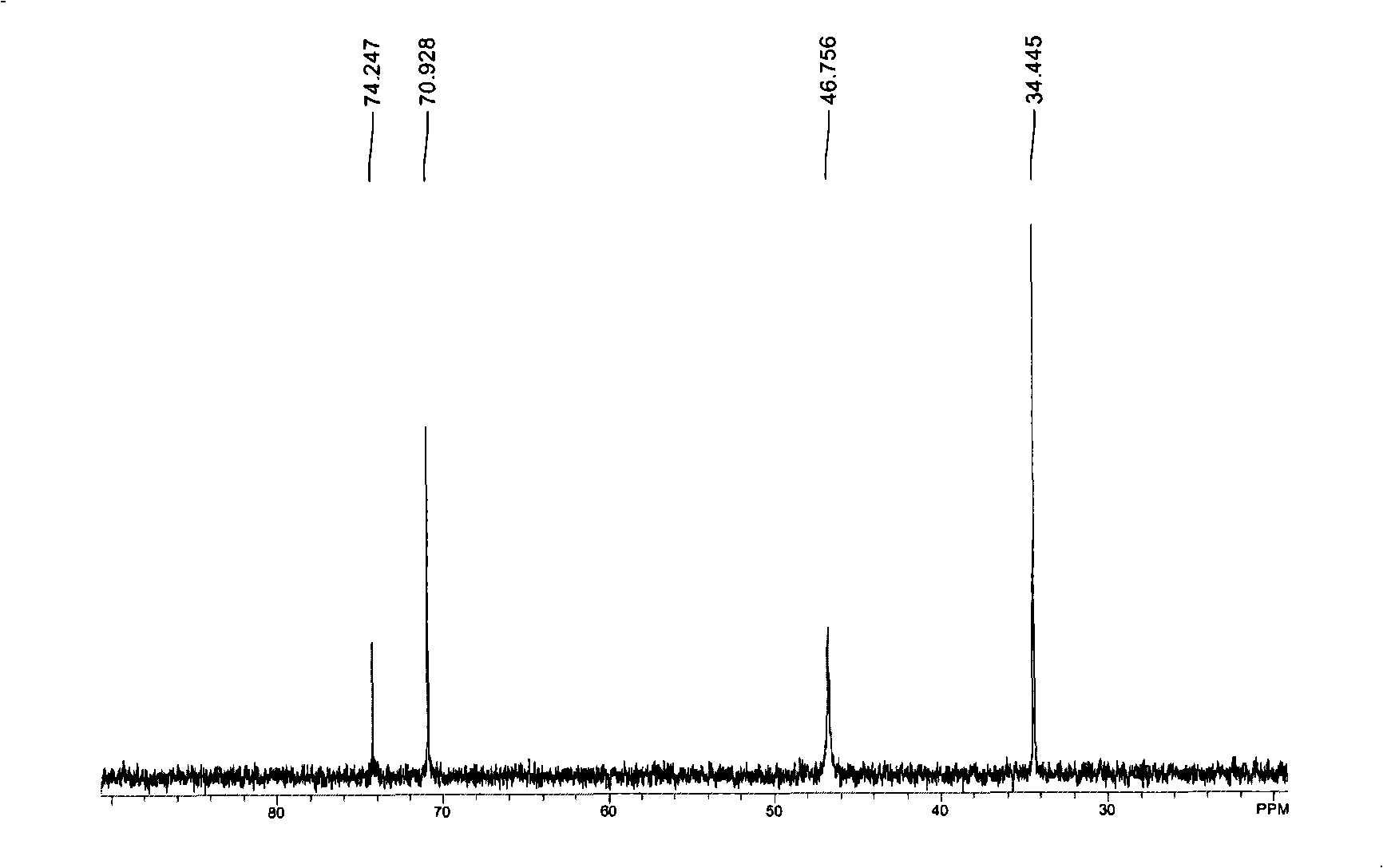
[0061] The Sn(dmamb) prepared in embodiment 3 2 compound 1 H-NMR is shown in Figure 5 middle.
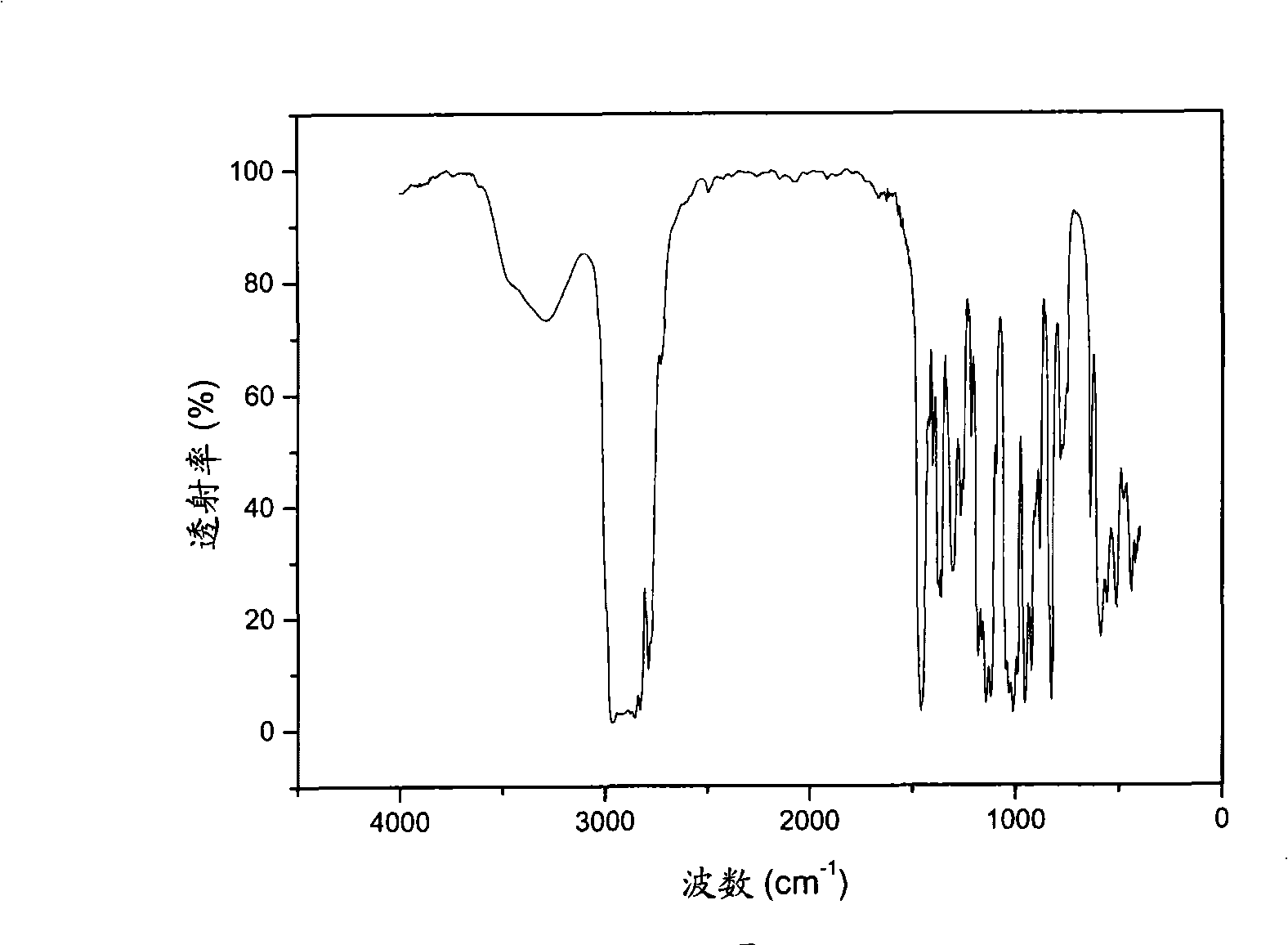
[0062] The Sn(dmamb) prepared in embodiment 3 2 The FT-IR of the compound is sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com