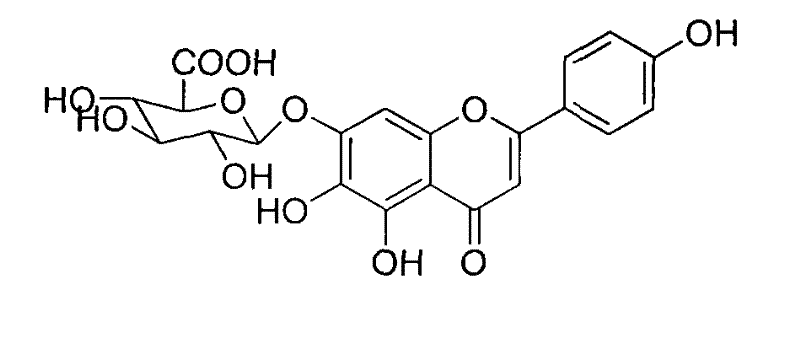
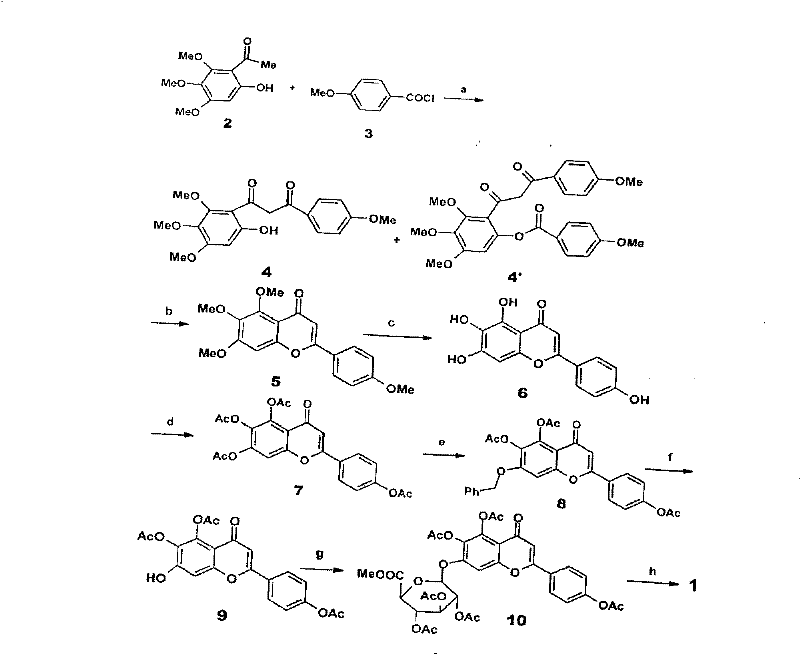
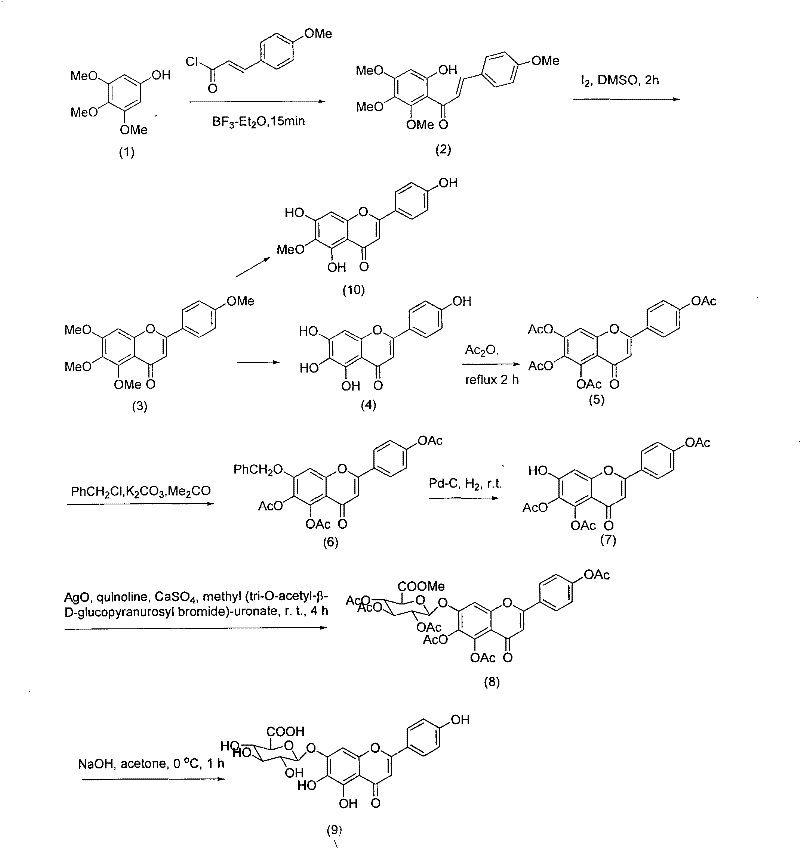
Method for synthesizing 5,6,4'-trihydroxyflavone-7-O-D-glucuronic acid
A technology of glucuronic acid and trihydroxyflavone, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of long synthetic routes and low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Take 1.84g of 3,4,5-trimethoxyphenol, 2.7g of p-methoxycinnamic acid chloride, put into 20mL BF 3 -Et 2 O solution, make it fully dissolved, add 2g 4A molecular sieves, heat and reflux for 15 minutes, stop heating, filter, and recrystallize with petroleum ether-ethyl acetate (3:1) to obtain 9-hydroxyl-5,6,7,4 '-tetramethoxychalcone 3.13g, the transfer rate of this step is 91%.
[0017] Take 3.44g of 9-hydroxy-5,6,7,4'-tetramethoxychalcone, 200mg of iodine, dissolve in 25mL of DMSO, reflux for 2 hours, then carefully pour into 200g of crushed ice, filter and precipitate with 20% Na 2 SO 3 Wash and recrystallize with petroleum ether-ethyl acetate (10:1) to obtain 2.98 g of 5,6,7,4'-tetramethoxyflavone with a transfer rate of 87%.
[0018] Take 3.42g of 5,6,7,4'-tetramethoxyflavone, add 5ml of 47% HBr, 10ml of glacial acetic acid, mix well, heat to reflux for 18 hours, then pour 200g of crushed ice carefully to obtain a yellow precipitate. The precipitate was collecte...
Embodiment 2
[0025] Purification of target product
[0026] Take 100g of the finished product, add 1000mL of pure water, adjust the pH value to 7 with 30% sodium hydroxide solution, make it completely dissolved, filter, add 8 times acetone to the filtrate at 25°C for precipitation, and stir while adding acetone , to make the precipitation complete, let stand for 12 hours, filter, add acetone to wash three times, then move the precipitate to another container, add 6 times the amount of 40% acetone, stir well, then add 25% hydrochloric acid to adjust the pH value to 1 ~2, let stand for 10 hours, filter with suction, wash with water until neutral, wash with ethanol once, and dry to obtain the refined product. According to HPLC analysis, the content of 5,6,4'-trihydroxyflavone-7-O-D-glucuronic acid in the obtained sample was 99.25%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com