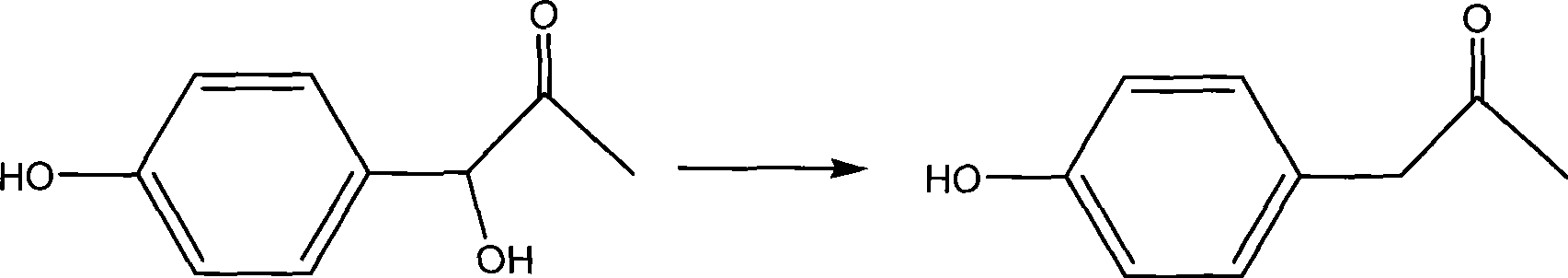
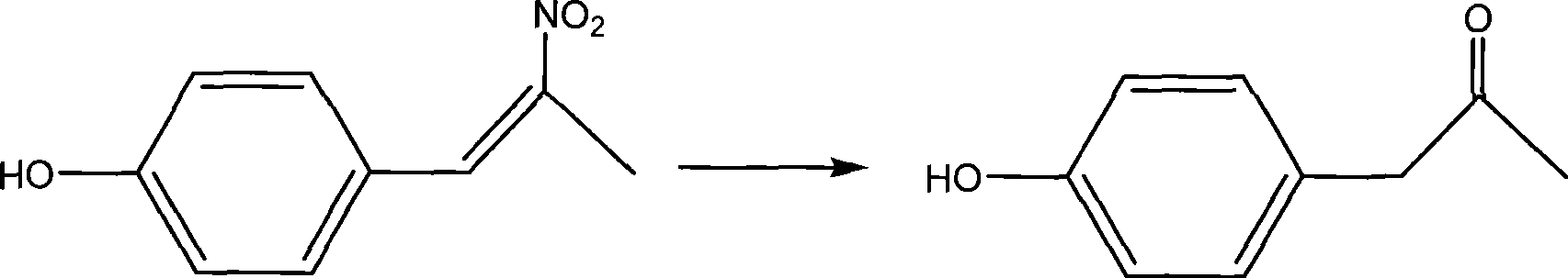
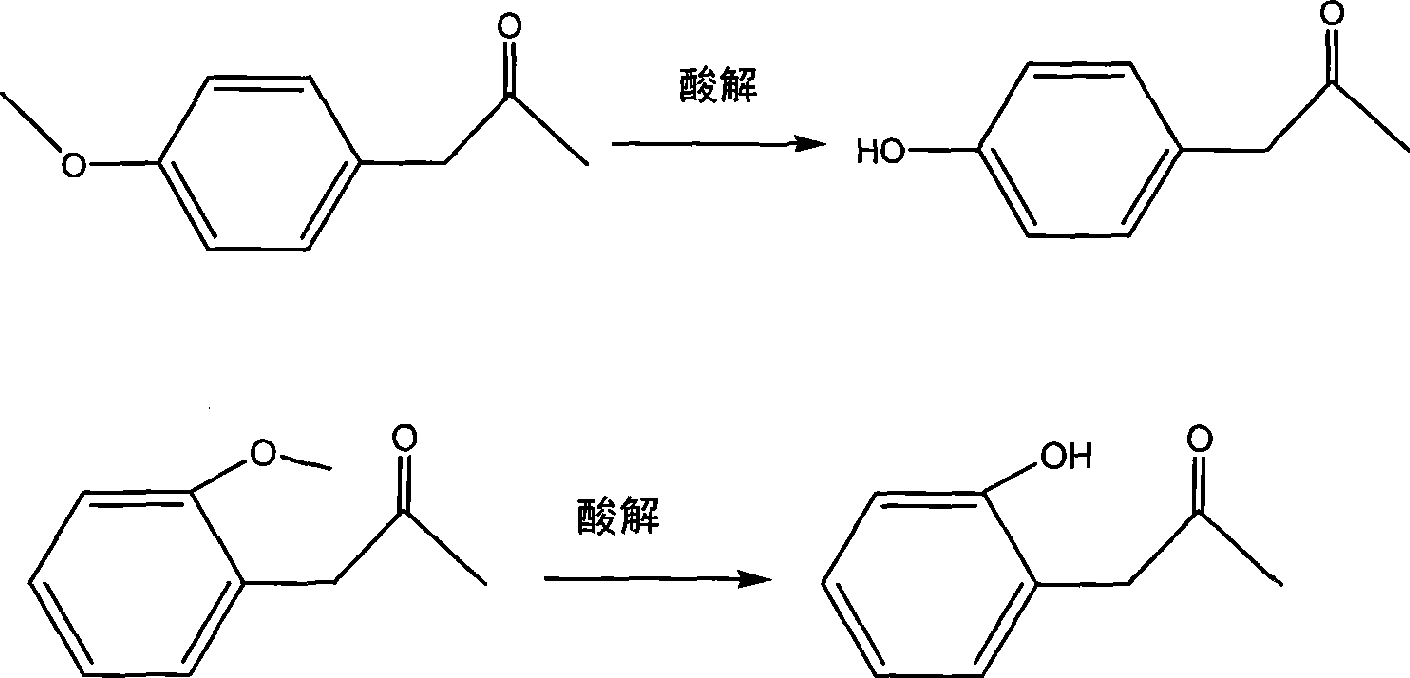
Synthesis method of medical midbodies of para(ortho)-hydroxybenzoic acetone
A technology of o-hydroxyphenylacetone and hydroxyphenylacetone, which is applied in the field of synthesis of pharmaceutical intermediates p-hydroxyphenylacetone and o-hydroxyphenylacetone, can solve problems such as difficulty in obtaining raw materials, and achieves low production cost and easy industrialization , The effect of low process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 47 grams of p-methoxyphenylacetone, 100 milliliters of 48% hydrobromic acid, and 200 milliliters of glacial acetic acid in a 1000 milliliter three-necked flask, slowly heat up to reflux, TLC tracking (developer: petroleum ether: ethyl acetate=4: 1) Stop the reaction after reacting to the point where there is no raw material, cool and concentrate under reduced pressure, then add 100 ml of water, then extract the water phase 4 times with 100 ml of ethyl acetate, combine the organic layers, wash the ester layer with saturated aqueous sodium chloride solution, After drying overnight with anhydrous sodium sulfate, after concentrating and refining, 35.5 grams of the product were obtained, with a yield of 82.6%, and the gas phase detection result: the content was greater than 99.5%. Properties: light yellow crystal, melting point 34-35 degrees, bp177 degrees / 11mmHg.
Embodiment 2
[0022] Add 47 grams (0.287mol) of p-methoxyphenylacetone in a 1000 milliliter three-necked flask, 200 milliliters of 36% concentrated hydrochloric acid, 200 milliliters of glacial acetic acid, slowly warming up to reflux, TLC traces (developing agent: sherwood oil: ethyl acetate =4: 1) Stop the reaction after reacting to the point of no raw material, cool and concentrate under reduced pressure, then add 100 ml of water, then extract the aqueous phase with 100 ml of ethyl acetate for 4 times, combine the organic layers, and wash with saturated aqueous sodium chloride solution The ester layer was dried overnight with anhydrous sodium sulfate, concentrated and then refined to obtain 30.2 g of the product with a yield of 70.2%. Properties: light yellow crystal, melting point 34-35 degrees, bp177 degrees / 11mmHg.
Embodiment 3
[0024] Add 47 grams (0.287mol) of o-methoxyphenylacetone in a 1000 milliliter three-necked flask, 100 milliliters of 48% hydrobromic acid, 200 milliliters of glacial acetic acid, slowly warming up to reflux, TLC tracking (developing agent: sherwood oil: ethyl acetate Ester=4:1) Stop the reaction after reacting to the point where there is no raw material, cool and concentrate under reduced pressure, then add 100 ml of water, then extract the aqueous phase with 100 ml of ethyl acetate for 4 times, combine the organic layers, and wash with saturated aqueous sodium chloride solution The ester layer was washed, dried overnight with anhydrous sodium sulfate, concentrated and refined to obtain 31.6 g of light yellow crystals, with a yield of 73.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com