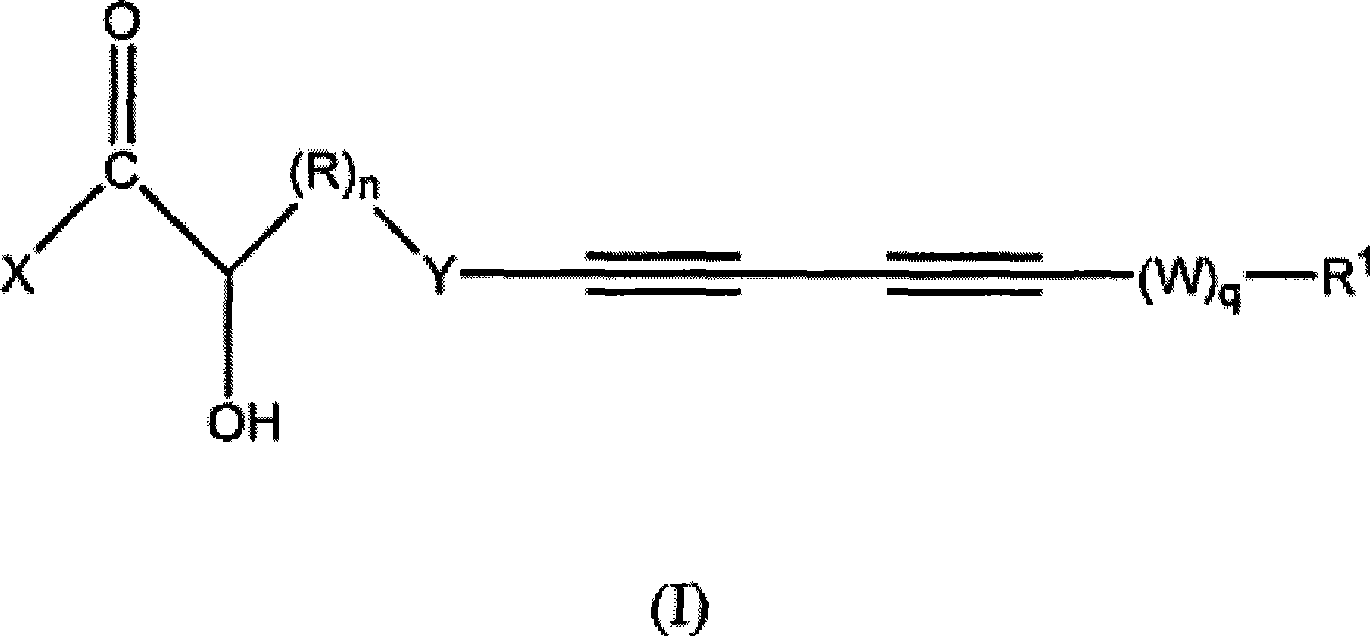
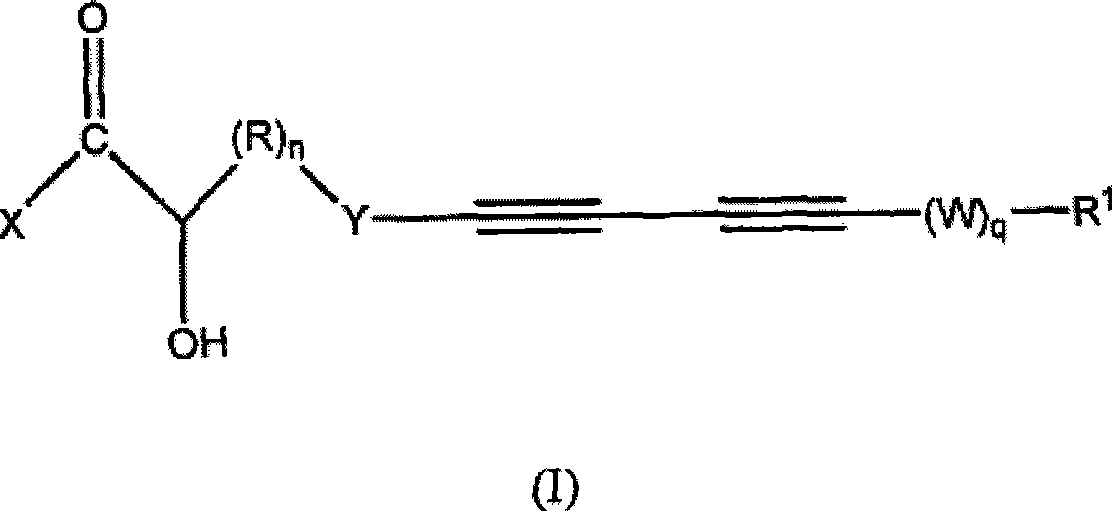
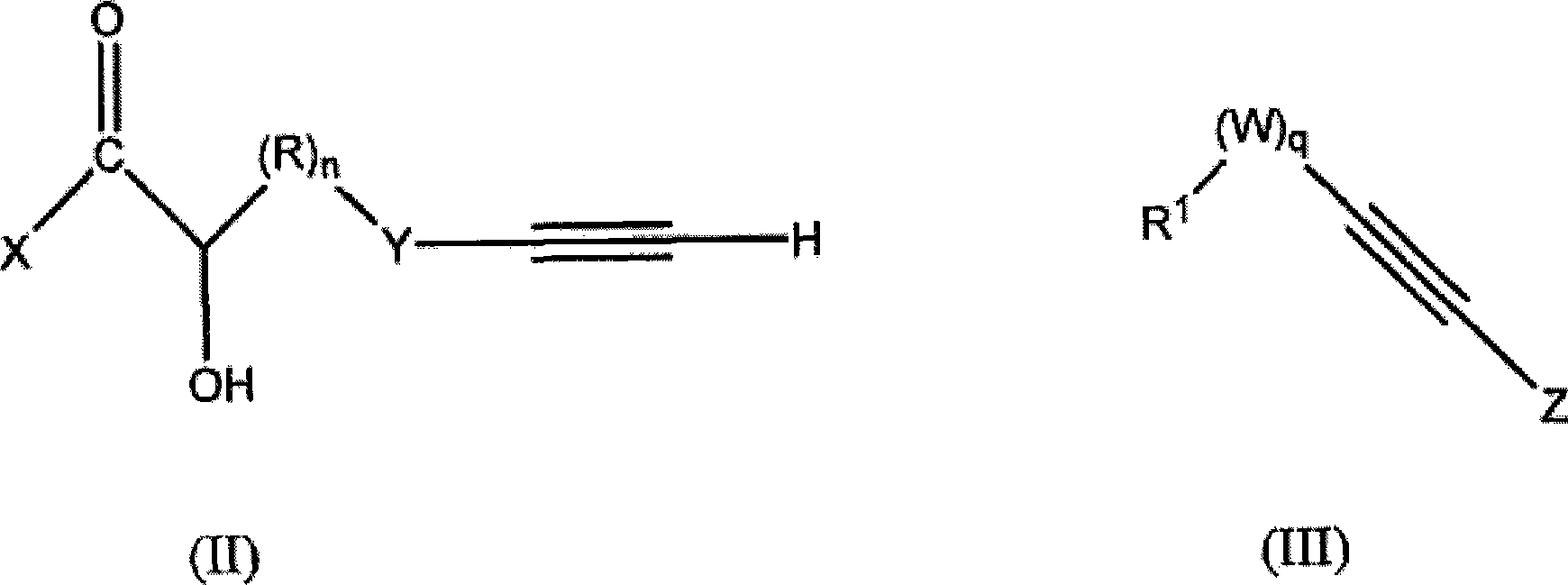Acetylenic compounds
A compound and selected technology, applied in the field of acetylenic compounds, can solve the problem of PDA color transformation that is not fully understood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0272] Part A: 11-Trimethylsilyl-undec-10-ynoic acid
[0273]
[0274] Use n-BuLi (1.6M hexane solution, 37.72ml, 60.35mmol, 2.2 molar equivalent) to -78 ℃ undec-10-ynoic acid (5g, 27.43mmol) in anhydrous THF solution (200ml) deal with. After stirring for 2 minutes, trimethylsilyl chloride (10.76ml, 85.04mmol, 9.24g, 3.1 molar equivalent) was added. The reaction mixture was slowly warmed to 25°C and stirred for 1 hour. The reaction was quenched by adding 2N HCl aqueous solution (50ml), and using CH 2 Cl 2 (3×50ml) extraction. Na for organic layer 2 SO 4 Dry, filter and concentrate. Column chromatography (SiO 2 , EtOAc-hexane 2:1) to obtain the acid in the form of a white solid (5.3g, 20.83mmol, 76%): 1 H-NMR(CDCl 3 , 400MHz): δ [ppm] = 0.61 (9H, s), 1.17-1.39 (8H, m), 1.41-1.53 (2H, m), 1.55-1.68 (2H, m), 2.17 (2H, t, J =6.5Hz), 2.35(2H, t, J=6.5Hz)
[0275] Part B: Synthesis of 2-hydroxy-11-trimethylsilyl-undec-10-ynoic acid
[0276]
[0277] Dry diisopropylamine (6.71ml, 4...
Embodiment 2
[0295] Part A: Synthesis of pentadec-8,10-diyn-1-ol
[0296]
[0297] In a nitrogen atmosphere, to a stirred solution of 1-iodo-1-hexyne (6.2g, 29.80mmol) and 8-nonyn-1-ol (2.46g, 17.53mmol) in pyrrolidine (50ml) was added iodide Copper (I) (2.98 mmol, 0.57 g). After stirring for 30 minutes at room temperature, the mixture was hydrolyzed with saturated aqueous ammonium chloride solution and extracted with diethyl ether. MgSO for organic extracts 4 Dry and remove the solvent in vacuo. Column chromatography (SiO 2 , EtOAc-hexane: 2:1) to obtain 3.35 g (15.19 mmol, 87%) of pure pentadec-8,10-diyn-1-ol.
[0298] 1 H-NMR(CDCl 3 , 400MHz): δ [ppm] = 0.89 (3H, t, J = 7.3 Hz), 1.26-1.62 (14H, m), 2.24 (4H, t, J = 6.6 Hz), 3.62 (2H, t, J = 7.0Hz)
[0299] Part B: Synthesis of 15-iodo-pentadec-5,7-diyne
[0300]
[0301] At -10°C, the CH of pentadec-8,10-diyn-1-ol (2.6g, 11.80mmol), triphenylphosphine (3.4g, 12.98mmol) and imidazole (0.96g, 14.16mmol) 2 Cl 2 (60ml) Add a small amount of...
Embodiment 3
[0324] Part A: 2-hydroxy-heptadecene-10,12-diyl acid crystal
[0325] In a vacuum, at 40°C, reduce the volume of a methanol solution (30ml) of 2-hydroxy-heptadecene-10,12-diynoic acid (100mg, 0.36mmol) to 1ml, and add a tetrahydrofuran solution of hydrochloric acid to it (20ml 1M aqueous HCl in 20ml THF). The solution was stirred overnight at room temperature, then the solvent was removed to obtain a clear oil. It was dissolved in dichloromethane (1ml), and then hexane was introduced by slow vapor diffusion in a closed system at -10°C. After 3 days, well-formed crystals of 2-hydroxy-heptadediynoic acid appeared in dichloromethane.
[0326] Part B: Polymerization of 2-Hydroxy-Heptadecene-10,12-Diynoic Acid Single Crystal
[0327] Take several single crystals prepared as described in Part A above and expose them to UV radiation (254 nm) from a small hand-held lamp. After a few seconds of exposure, the crystals turned dark blue. When exposed to organic solvents or high temperatures, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com