Magnetic nanometer material for processing organic pollutants
An organic pollutant and magnetic nanotechnology, applied in the fields of nanomaterials and environmental science, can solve the problems of difficult large-scale extraction, high cost, variability, etc., and achieve high catalytic efficiency, good thermal stability, and no secondary pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
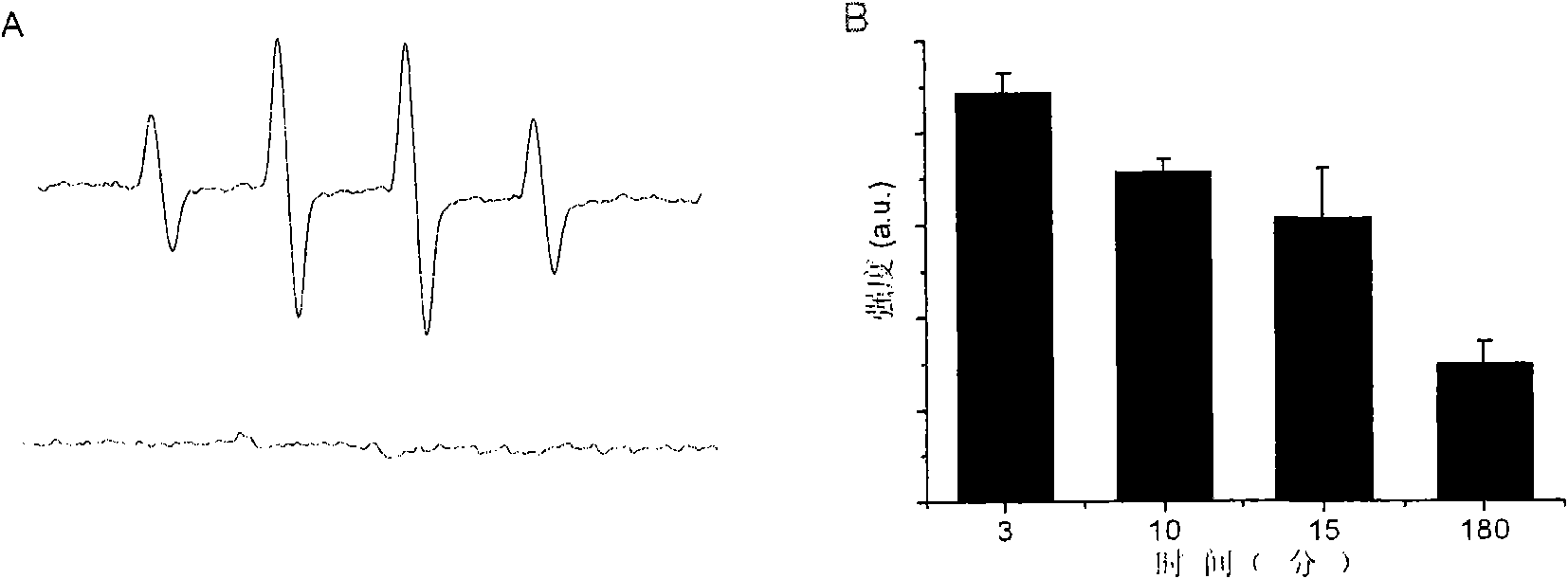
[0025] Example 1. Magnetic nanomaterials can catalyze H 2 o 2 Generate OH free radicals.
[0026] Reagent: 5,5'-dimethyl-1-pirroline-N-oxide (DMPO) was purchased from Sigma-Aldrich Inc (USA). 30% H 2 o 2 and phenol and Fe 3 o 4 Synthetic materials of magnetic nanoparticles (ammonia water, ferric chloride hexahydrate) were purchased from Beijing Chemical Reagent Company. The magnetic nanomaterials used are synthesized by hydrothermal method (see Ma, M., et al.J Magn Mater, 268, 33 (2004) and Sun, Y.-k., et al.Colloids and Surfaces A, 245, 15(2004)).
[0027] Method: Take 12μg Fe 3 o 4 Magnetic nanomaterials (particle size 13nm), add 125 μl pH3.0 deionized water, add 4 μl phenol (100 mM, soluble in water) and 1 μl 30% H 2 o 2 . At different reaction times, 40 μl of the reaction product was taken out and mixed with 10 μl of DMPO (500 mM), and the content of ·OH free radicals in the solution was measured by ESR.
[0028] Result: when Fe 3 o 4 magnetic nanomaterials a...
Embodiment 2
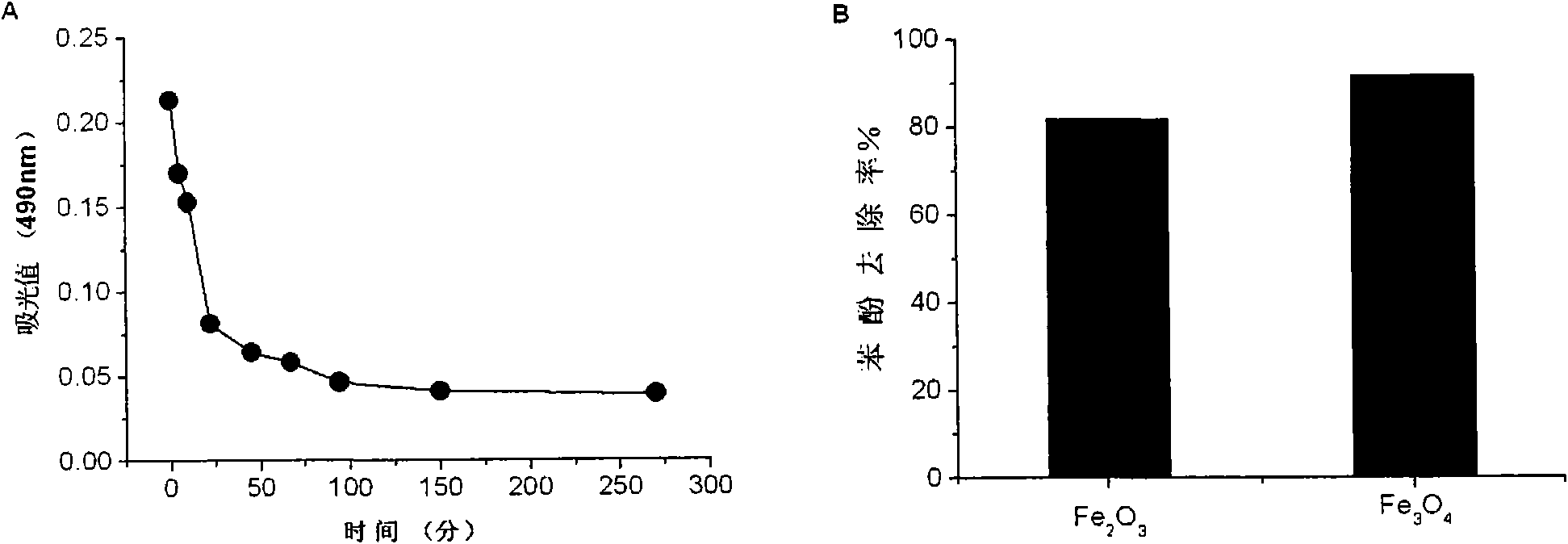
[0029] Example 2. Magnetic nanomaterials can catalyze the degradation of phenol.
[0030] Reagent: 30% H 2 o 2 , 4-aminoantipyridine (4-AAP), sodium bicarbonate (NaHCO 3 ), potassium ferricyanide (K 3 Fe(CN) 6 ) and phenol and Fe 3 o 4 Synthetic materials of magnetic nanoparticles (ammonia water, ferric chloride hexahydrate) were purchased from Beijing Chemical Reagent Company. The magnetic nanomaterials used are synthesized by hydrothermal method (see Ma, M., et al.J Magn Mater, 268, 33 (2004) and Sun, Y.-k., et al.Colloids and Surfaces A, 245, 15(2004)).
[0031] Method: Take 12μg Fe 3 o 4 and Fe 2 o 3Magnetic nanomaterials (particle size 13nm), were added to 125 μl of deionized water with pH 3.0, 4 μl of phenol (100 mM, soluble in water) and 1 μl of 30% H 2 o 2 . At different reaction times, remove 2 μl of the reaction product with 78 μl of NaHCO 3 (0.25M) was mixed and added to a 96-well ELISA plate. Then add 10 μl K 3 Fe(CN) 6 (83.4mM, dissolved in 0.25M...
Embodiment 3
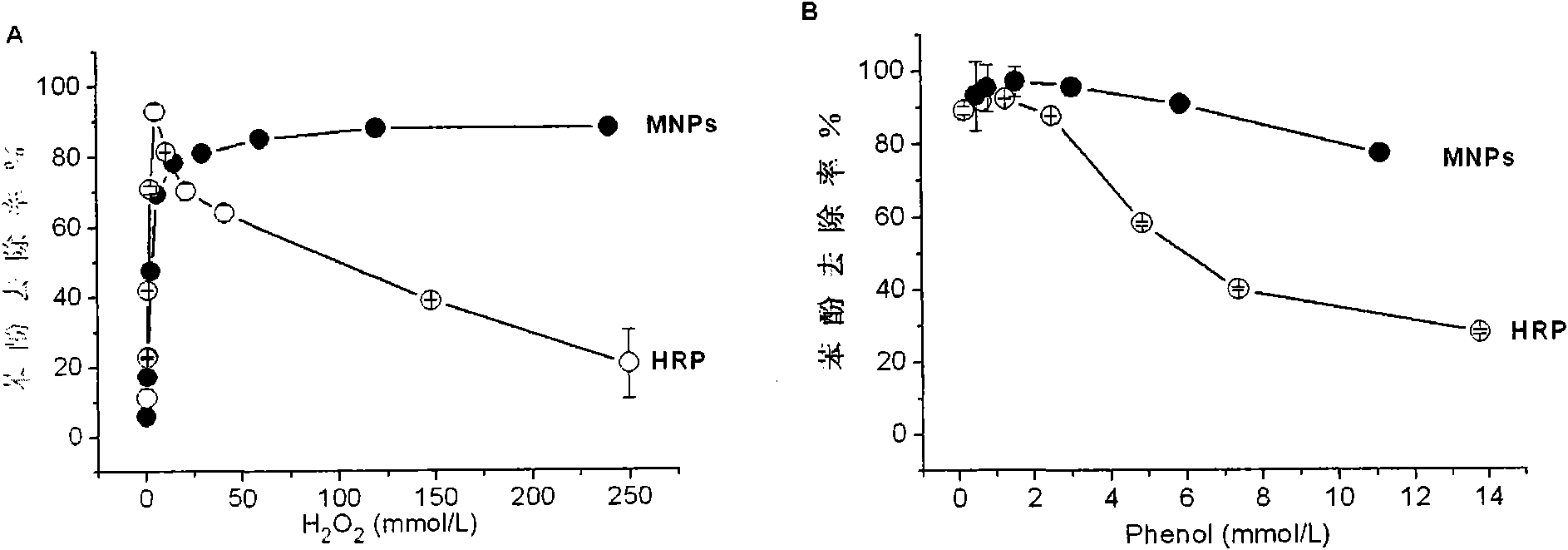
[0033] Example 3.H 2 o 2 Regulate Fe 3 o 4 Catalytic Efficiency of Magnetic Nanomaterials
[0034] Reagent: horseradish peroxidase (Horseradish Peroxidase, HRP, EC 1.11.1.7, >300 units / mg), purchased from Sigma-Aldrich Inc. (USA). 30%H 2 o 2 , 4-aminoantipyridine (4-AAP), sodium bicarbonate (NaHCO 3 ), potassium ferricyanide (K 3 Fe(CN) 6 ) and phenol and Fe 3 o 4 The synthetic materials of magnetic nanoparticles were purchased from Beijing Chemical Reagent Company.
[0035] Method: Take 12μg Fe 3 o 4 Magnetic nanomaterials (particle size 13nm) were added to 125 μl of deionized water with pH 3.0, and then 4 μl of phenol (100 mM, dissolved in water) was added. Another 1.5 μg of HRP was added to 125 μl of Tris-HCl buffer (0.1 M, pH 8.0), followed by 4 μl of phenol (100 mM, dissolved in water). Add varying amounts of 0.01 μl to 16 μl of 30% HO to each parallel reaction 2 o 2 , reacted for 3 hours. Take 2 μl of the reaction product to measure the phenol concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com