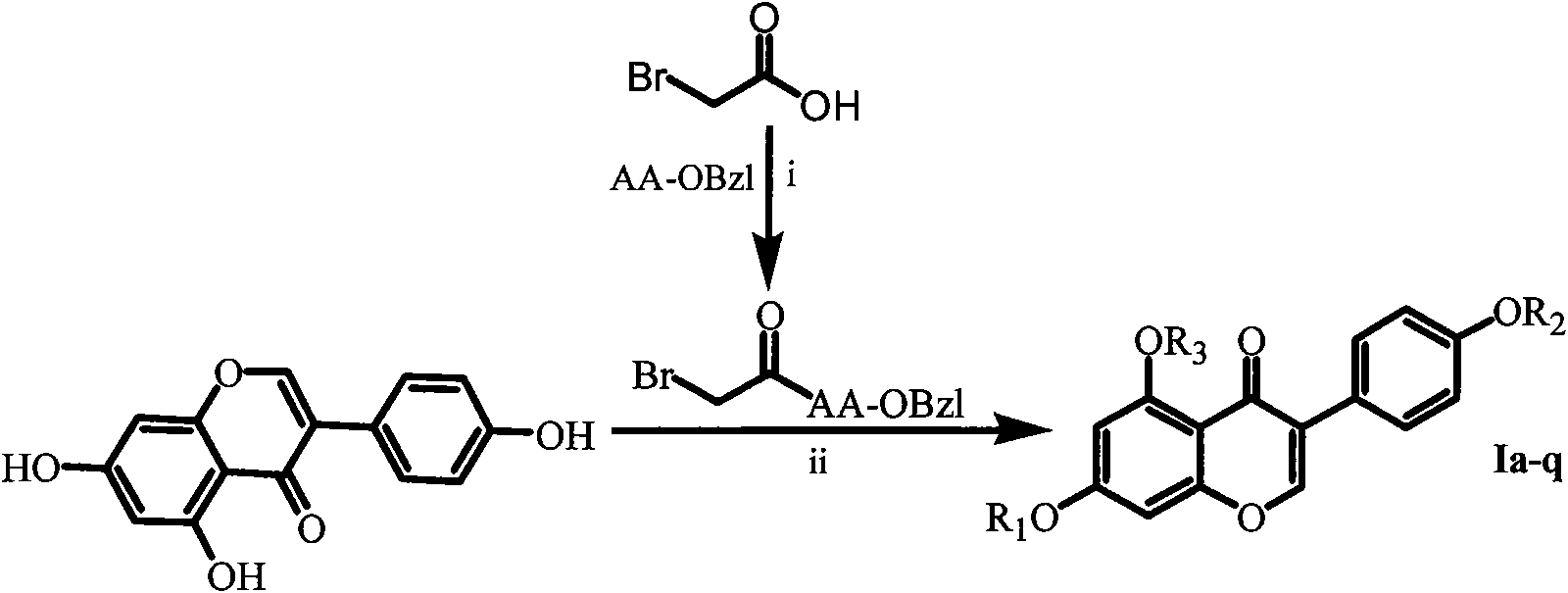
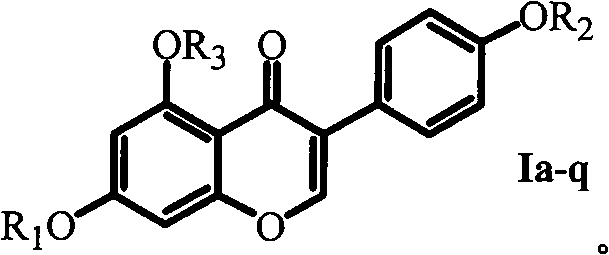
Isoflavone derivative modified by acetylaminoacid benzyl ester, preparation method and application thereof
A technology of amino acid benzyl ester and trihydroxy isoflavone, applied in the field of biomedicine, can solve the problem that the anti-tumor activity cannot meet the needs of anti-tumor treatment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 prepares the general method of bromoacetyl amino acid benzyl ester
[0017] Dissolve 0.005mol of bromoacetic acid with 60ml of anhydrous THF in a 100ml eggplant bottle, add 0.005mol of DCC under ice-cooling, wait until the reaction is complete (about 30 minutes), add 0.005mol of AA-OBzl, add NMM to adjust pH-8~9 , TLC to monitor the reaction, the reaction was terminated after the disappearance of the AA-OBzl raw material, filtered, and the filtrate was concentrated to dryness under reduced pressure. The obtained residue was dissolved in 100 mL of ethyl acetate, placed in a 250 mL separatory funnel, washed successively with saturated aqueous sodium bicarbonate (30 mL×3), saturated aqueous sodium chloride (30 mL×3), 5% potassium bisulfate Wash with aqueous solution (30mL×3), wash with saturated aqueous sodium chloride solution (30mL×3), wash with saturated aqueous sodium bicarbonate solution (30mL×3), and wash with saturated aqueous sodium chloride solution (...
Embodiment 2
[0018] Embodiment 2 prepares bromoacetylglycine benzyl ester
[0019] According to the general method of Example 1, 585 mg (yield 68.9%) of benzyl bromoacetylglycine was obtained as a colorless solid with 1 g of Tos·Gly-OBzl. ESI + -MS(m / z)310[M+H+Na] + .
Embodiment 3
[0020] Embodiment 3 prepares bromoacetylalanine benzyl ester
[0021] According to the general method of Example 1, 455 mg (yield 53.2%) of benzyl bromoacetylalanine was obtained as a colorless solid with 1 g of Tos·Ala-OBzl. ESI + -MS(m / z)324[M+H+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com