Method for preparing antiseptic triaryl-2-pyrazoline derivative by microwave
A technology of derivatives and fungicides, which is applied in the field of microwave synthesis of fungicides triaryl-2-pyrazoline derivatives, can solve the problems of difficult separation and purification, incomplete reaction, difficult homogeneous reaction, etc. The effect of convenient processing, improved reaction yield and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
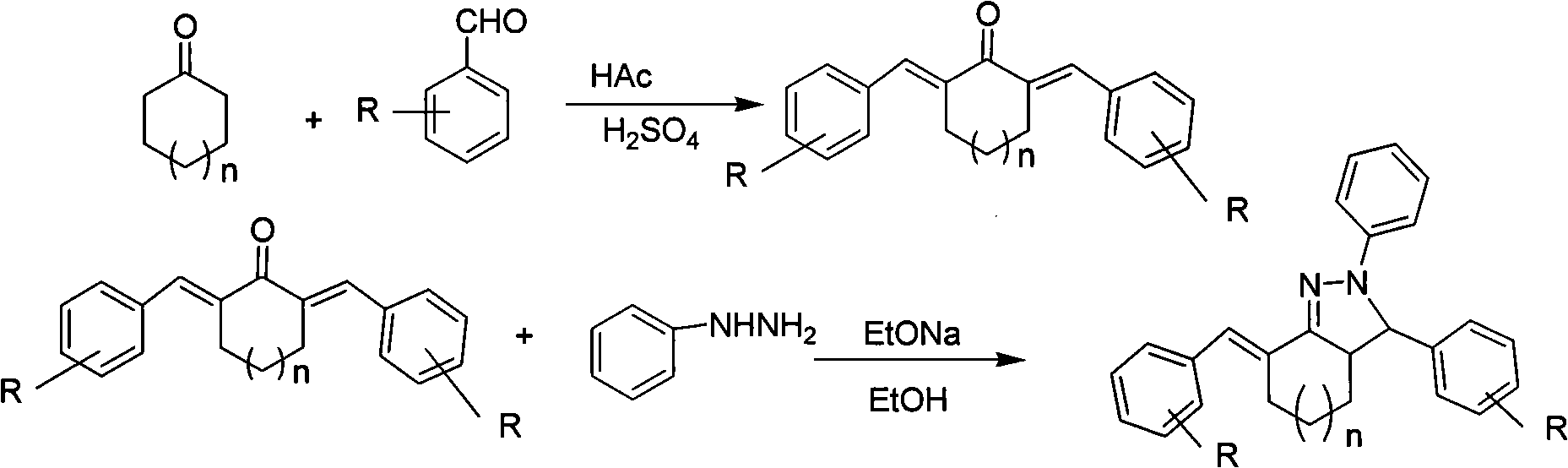
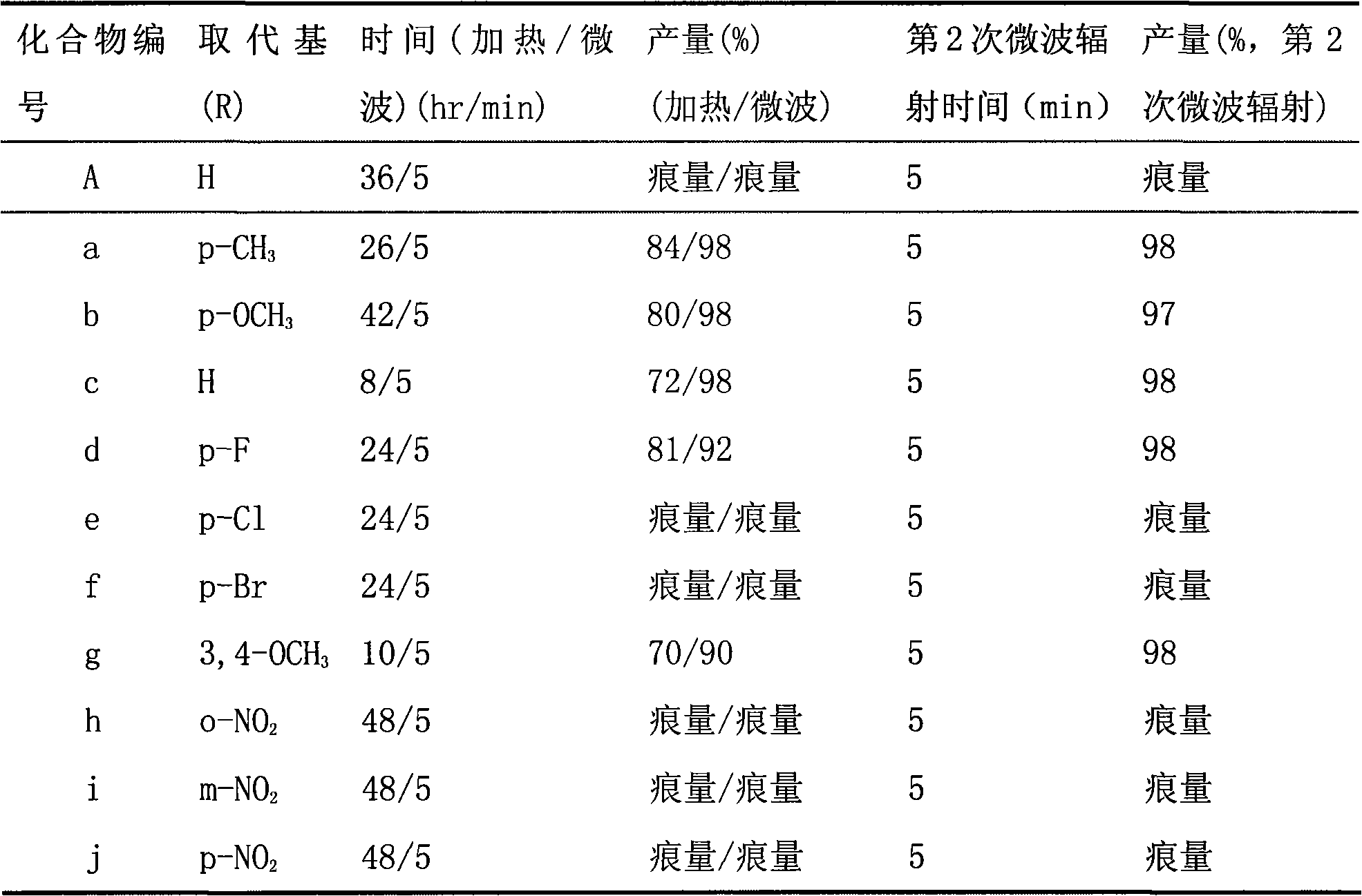
[0047] α, α'-dibenzylidene cyclohexanone is reacted with phenylhydrazine, and a triaryl-2-pyrazoline derivative (c) is synthesized under a conventional heating method.
[0048] In a 100ml three-neck flask, add 30ml of absolute ethanol, 3mmol α,α'-dibenzylidene cyclohexanone, heat, stir and dissolve, then add 6mmol of phenylhydrazine, 6mmol of sodium ethoxide, heat and reflux for 8 hours, and track the reaction to equilibrium by TLC . The reaction system was cooled in ice water, and a yellow solid precipitated out. The solid was filtered with suction and dried to obtain a crude product, which was recrystallized from methanol / dichloromethane to obtain a pure product. Yield: 72%.
[0049] C 26 h 24 N 2 , yellow solid, mp: 143-145°C, IR (KBr, cm -1 )3447, 3054, 2933, 2863, 1598, 1501, 1489, 1091, 1013. 1 HNMR (CDCl 3 , δppm) 1.44(1H, m), 1.65(1H, m), 1.91(1H, m), 2.19(1H, m), 2.42(1H, t), 2.98(1H, m), 3.03(1H, t ), 4.57 (1H, d), 6.81 (1H, t), 7.04 (2H, d), 7.15 (2H, t), 7....
Embodiment 2
[0051] α, α'-dibenzylidene cyclohexanone is reacted with phenylhydrazine, and the triaryl-2-pyrazoline derivative (c) is synthesized under the method of microwave radiation.
[0052]In a 150ml flask for microwave reaction, add 30ml absolute ethanol, 3mmol α, α'-dibenzylidene cyclohexanone, 6mmol phenylhydrazine, 6mmol sodium ethoxide, and react in a microwave oven with a microwave radiation power of 250W After 5 minutes, TLC followed the detection reaction to equilibrium. After the reaction was complete, the reaction system was cooled in ice water and a yellow solid precipitated out. The solid was filtered with suction and dried to obtain a crude product, which was recrystallized from methanol / dichloromethane to obtain a pure product. Yield: 98%. Compound characterization is the same as in Example 1.
Embodiment 3
[0054] α, α'-dibenzylidene cyclohexanone is reacted with phenylhydrazine, and a triaryl-2-pyrazoline derivative (c) is synthesized under the method of secondary microwave radiation.
[0055] In a 150ml flask used for microwave reaction, add 30ml absolute ethanol, 3mmol α, α'-dibenzylidene cyclohexanone, 6mmol phenylhydrazine, 6mmol sodium ethoxide, and react 5 Minutes, let the reaction mixture cool down to room temperature (20-25° C.), and then carry out the second microwave irradiation reaction. The reaction time of the second microwave radiation reaction was the same as that of the first microwave radiation reaction. Under the microwave radiation power of 250W, the reaction was carried out for 5 minutes, and the TLC tracking detection reaction reached equilibrium. After the reaction was complete, the reaction system was cooled in ice water and a yellow solid precipitated out. The solid was filtered with suction and dried to obtain a crude product, which was recrystallized fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com