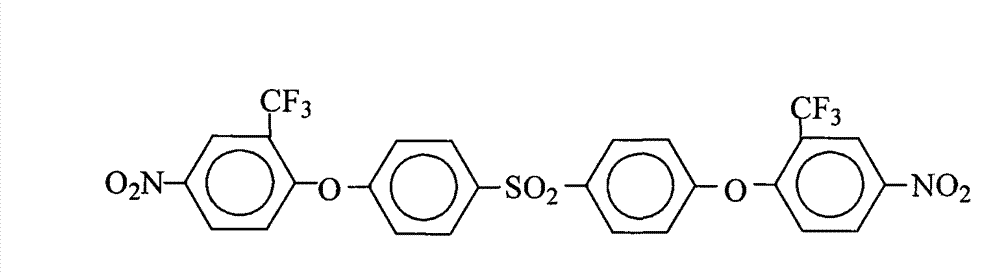
Method for preparing 4,4'-bis(4-nitro-2-trifluoromethylphenoxy)diphenylsulfone
A technology of trifluoromethylphenoxy, nitrotrifluorotoluene is applied in the field of preparation of aromatic fluorine-containing organic compounds, and can solve the problem of increasing raw material cost and production cost, increasing chemical equipment and personnel costs, and increasing waste liquid treatment. Costs and other issues, to achieve the effect of convenient source of raw materials, low cost, and fewer types of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
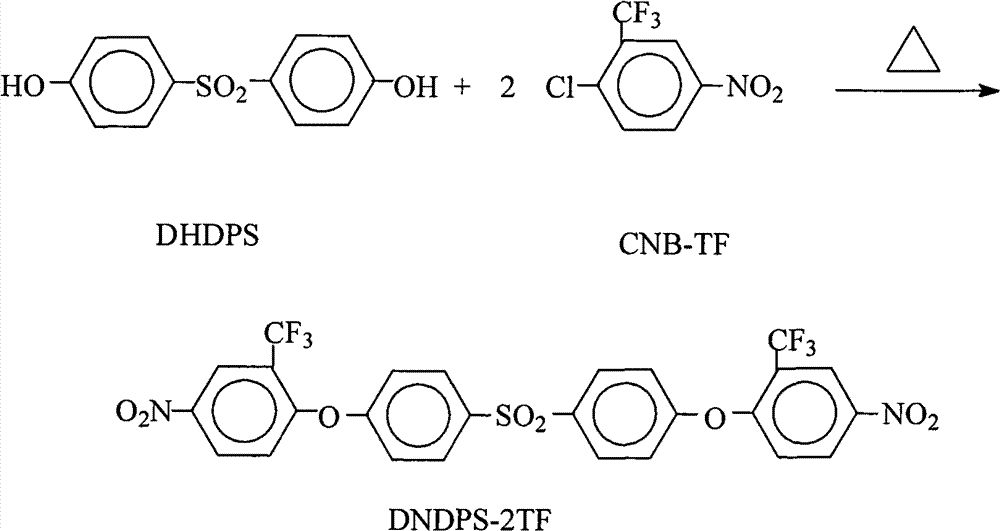
[0029] 25.0 grams (0.10 moles) of 4,4'-dihydroxydiphenylsulfone (DHDPS), 49.6 grams (0.22 moles) of 2-chloro-5-nitrotrifluoromethylbenzene (CNB-TF), 41.4 grams (0.30 mol) of potassium carbonate, 500 milliliters of N,N-dimethylformamide and 500 milliliters of toluene mixed solvent was put into the reaction kettle, after stirring at room temperature for 30min, heated to 110°C-130°C, refluxed for azeotropic water separation reaction for 15 After 1 hour, filter while it is hot, remove the filter residue, concentrate the mother liquor, control the solid content of the mother liquor in the range of 40%-80%, recover the solvent for recycling, cool and stand still, and precipitate milky white solid crystalline products, filter, and use pure water Wash 2~3 times, dry, obtain 61.7 grams of 4,4 '-bis(4-nitro-2-trifluoromethylphenoxy group) diphenyl sulfone (DNDPS-2TF) milky white solid product (theoretical yield is 62.8 gram), the purity is 99.8%, and the melting point is 198.4°C-199.2°C...
Embodiment 2
[0031] 25.0 grams (0.10 moles) of 4,4'-dihydroxydiphenylsulfone (DHDPS), 47.4 grams (0.21 moles) of 2-chloro-5-nitrotrifluoromethylbenzene (CNB-TF), 41.4 grams (0.30 Mole) salt of wormwood, 10.6 grams (0.10 mole) sodium carbonate, 479 milliliters of N, the mixed solvent of N-dimethylacetamide, 100 milliliters of N-methyl-2-pyrrolidone and 58 milliliters of xylenes are put into reaction kettle, After stirring at room temperature for 30 minutes, heat to 140°C-150°C, reflux for azeotropic water separation for 14 hours, filter while hot, remove the filter residue, concentrate the mother liquor, so that the solid content of the mother liquor is controlled within the range of 40%-80%, Recover the solvent for recycling, cool and stand still, and precipitate a milky white solid crystalline product, filter, wash with pure water 2 to 3 times, and dry to obtain 60.7 grams of 4,4'-bis(4-nitro-2-trifluoroform phenoxy)diphenyl sulfone (DNDPS-2TF) milky white solid product (theoretical yield...
Embodiment 3
[0033]25.0 grams (0.10 moles) of 4,4'-dihydroxydiphenylsulfone (DHDPS), 45.1 grams (0.20 moles) of 2-chloro-5-nitrotrifluoromethylbenzene (CNB-TF), 27.6 grams (0.20 mol) potassium carbonate, 100 milliliters of dimethyl sulfoxide, 181 milliliters of N-methyl-2-pyrrolidone, 100 milliliters of o-dichlorobenzene and 160 milliliters of xylene mixed solvents were put into the reaction kettle, after stirring at room temperature for 30min, Heating to 140°C-170°C, refluxing and azeotropic water separation for 12 hours, filtering while hot, removing the filter residue, concentrating the mother liquor so that the solid content of the mother liquor is controlled within the range of 40%-80%, and recovering the solvent for recycling. Cooling and standing still, the milky white solid crystalline product was precipitated, filtered, washed 2 to 3 times with pure water, and dried to obtain 61.0 grams of 4,4'-bis(4-nitro-2-trifluoromethylphenoxy) di Phenylsulfone (DNDPS-2TF) milky white solid pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com