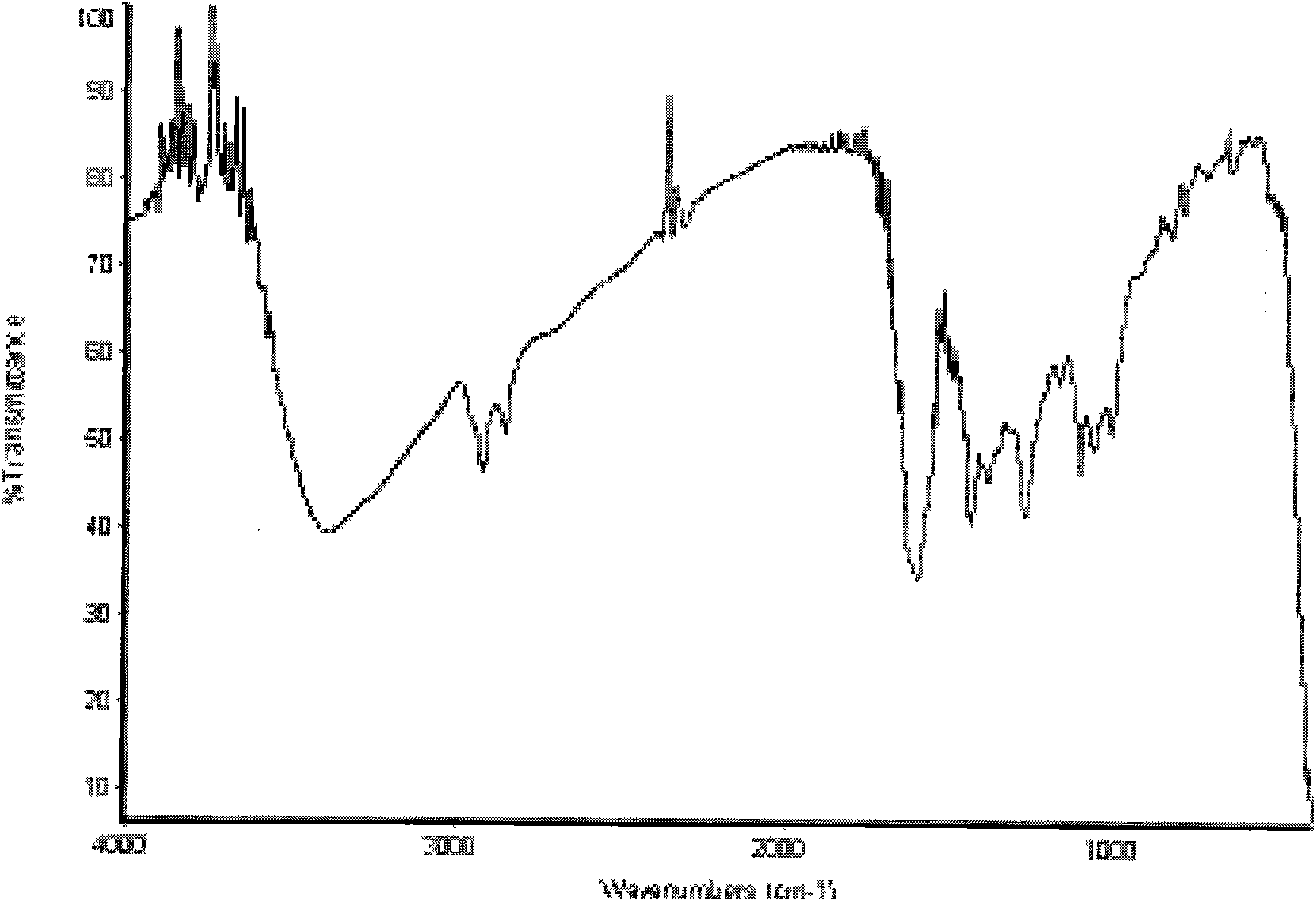
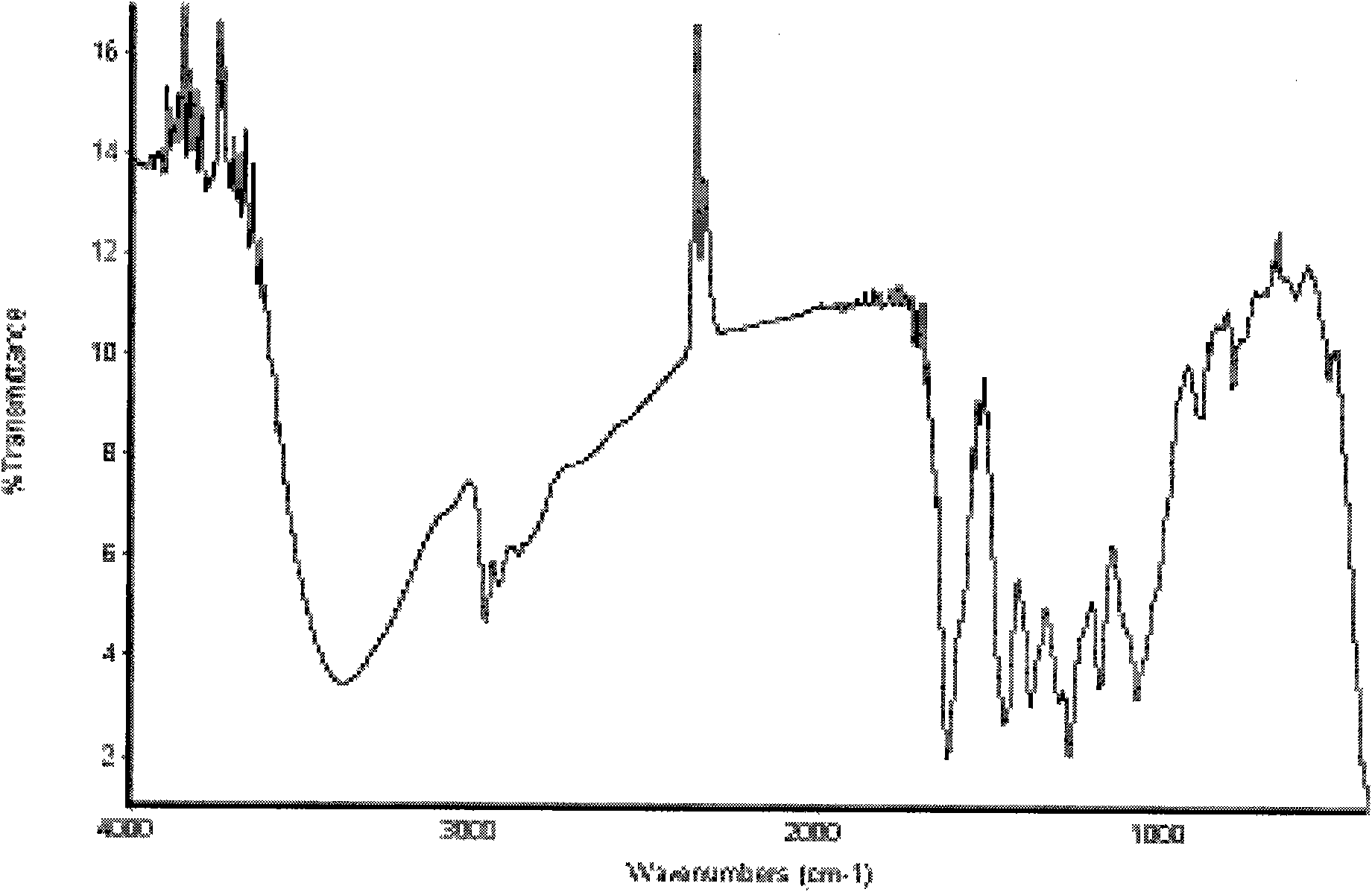
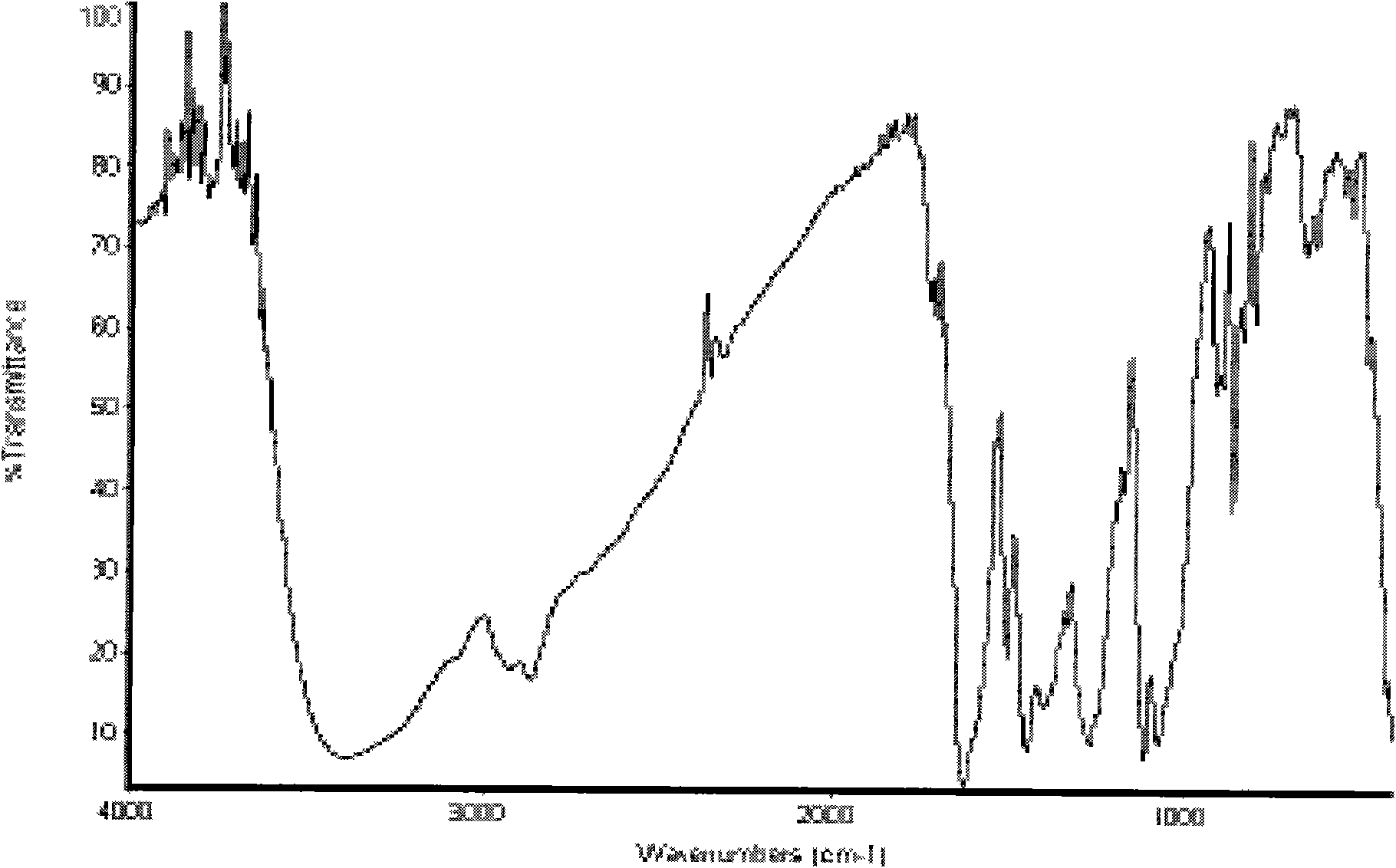
Synthetic method of puerarin derivatives
A technology of puerarin derivatives and synthesis methods, which is applied in the direction of sugar derivatives, drug combinations, cardiovascular system diseases, etc., can solve the problems of reducing biological activity, etc., and achieve the effects of simple operation, high yield, and easy industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0019] The synthesis of embodiment 13'-methylene-dimethylamine-puerarin
[0020] Dimethylamine hydrochloride first undergoes Mannich reaction with formaldehyde to generate methylene-diimine, and then methylene-diimine undergoes condensation reaction with puerarin phenolic hydroxyl group ortho to generate 3′-methylene-dimethylamine-pueraria white.
[0021] (1) Preparation of dimethylamine aqueous solution
[0022] Accurately weigh 30g dimethylamine hydrochloride (CH 3 )2NH.HCL, and then add a certain amount of distilled water to prepare a 50-70% aqueous solution;
[0023] (2) Synthesis of two-(dimethylamino)methane
[0024] Under cooling in an ice bath, add 5-25ml of 37%-40% formaldehyde aqueous solution to a 100mL three-neck round-bottomed flask, and while stirring, add 0.25-0.45mol of dimethylamine aqueous solution (add dropwise with a dropping funnel, Keep the reaction temperature lower than 50°C), dropwise is completed, stir at room temperature for 15-45 minutes, then a...
Embodiment 23
[0033] Embodiment 23', the synthesis of 5'-methylene-diethylamine-puerarin
[0034] Diethylamine first undergoes Mannich reaction with formaldehyde to generate methylene-diethylamine, and then methylene-diethylamine undergoes condensation reaction with puerarin phenolic hydroxyl group ortho to generate 3′, 5′-methylene-diethylamine - Puerarin.
[0035] (1) Synthesis of two-(diethylamine) methane
[0036] In a three-necked round-bottomed flask, add 50-85mL of 36%-40% formaldehyde aqueous solution, add 80-140g of diethylamine dropwise under the condition of cooling in an ice bath, and keep the reaction temperature at 15°C-35°C. After the dropwise addition is completed, React at room temperature for 2-6 hours, the solution appears to be stratified, the upper layer is an organic layer, and the lower layer is a water layer. Move the solution to a separatory funnel, separate the upper organic layer, dry it with anhydrous sulfuric acid, place it, filter it, and then conduct normal ...
Embodiment 33
[0045] The synthesis of embodiment 3'-methylene-morpholine-puerarin
[0046] Morpholine first undergoes a Mannich reaction with formaldehyde to generate methylene-morpholine, and then methylene-morpholine undergoes a condensation reaction with puerarin at the ortho position of the phenolic hydroxyl group to generate 3′-methylene-morpholine-puerarin.
[0047] (1) Synthesis of 4-4'-methylenebismorpholine
[0048] Add 20-50g morpholine into a three-neck round bottom flask, slowly add 10-25g 36%-40% formaldehyde solution dropwise with a dropping funnel, and at the same time cool in a water bath to control the reaction temperature not exceeding 50°C, after the dropwise addition, stir at room temperature After standing for 0.5-3.5 hours, the solution appears to be stratified. The upper layer is organic matter, and the lower layer is water. Move it to a separatory funnel to separate the upper layer, add anhydrous potassium carbonate to dry, filter, then distill under reduced pressure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com