Kirenol derivative and application thereof in preparation of proinflammatory cytokine inhibitor
A physiological and compound technology, applied in the direction of drug combination, active ingredients of hydroxyl compounds, medical preparations containing active ingredients, etc., can solve the problems of not affecting the process of joint damage, long-term tolerance and limited effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
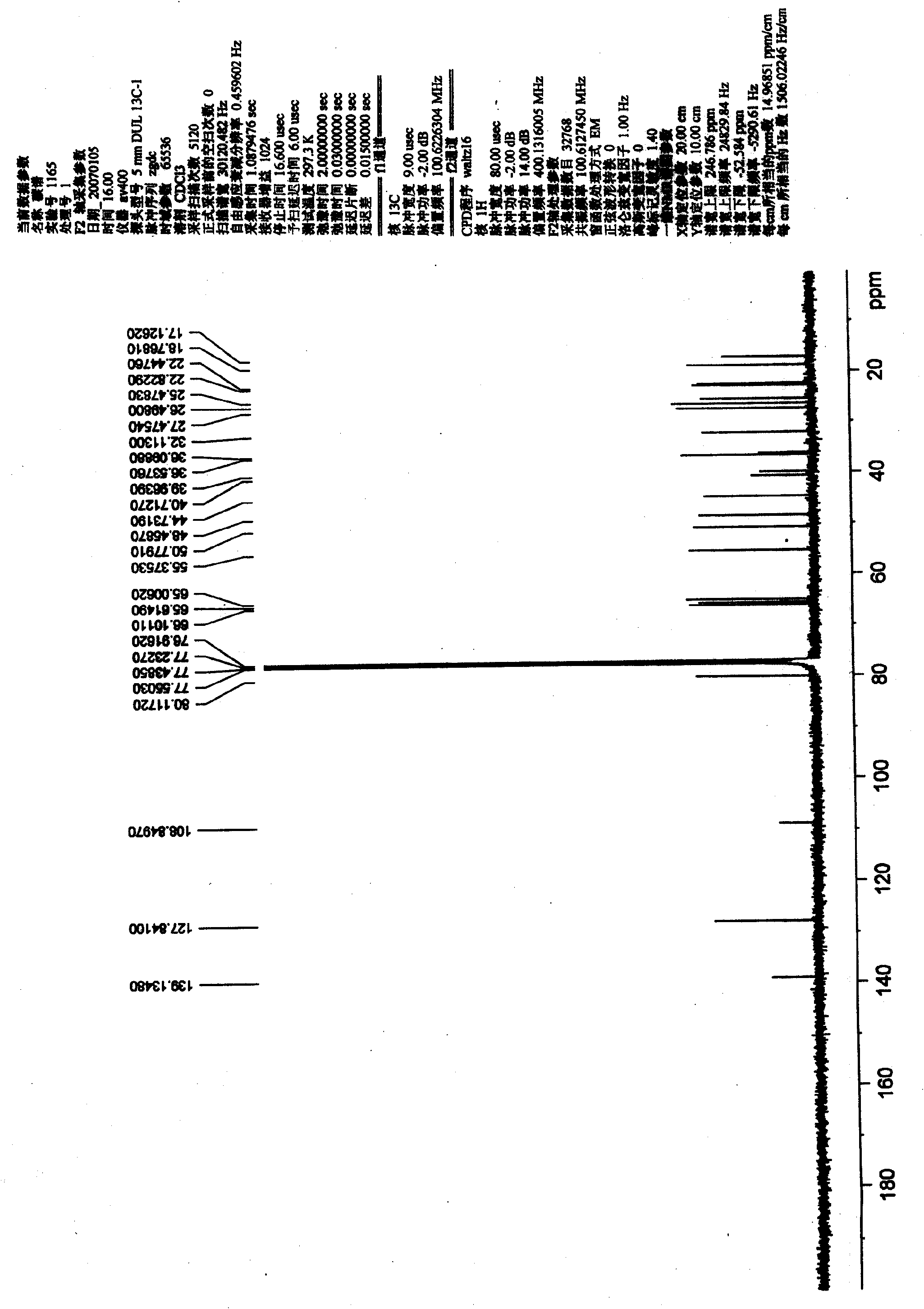
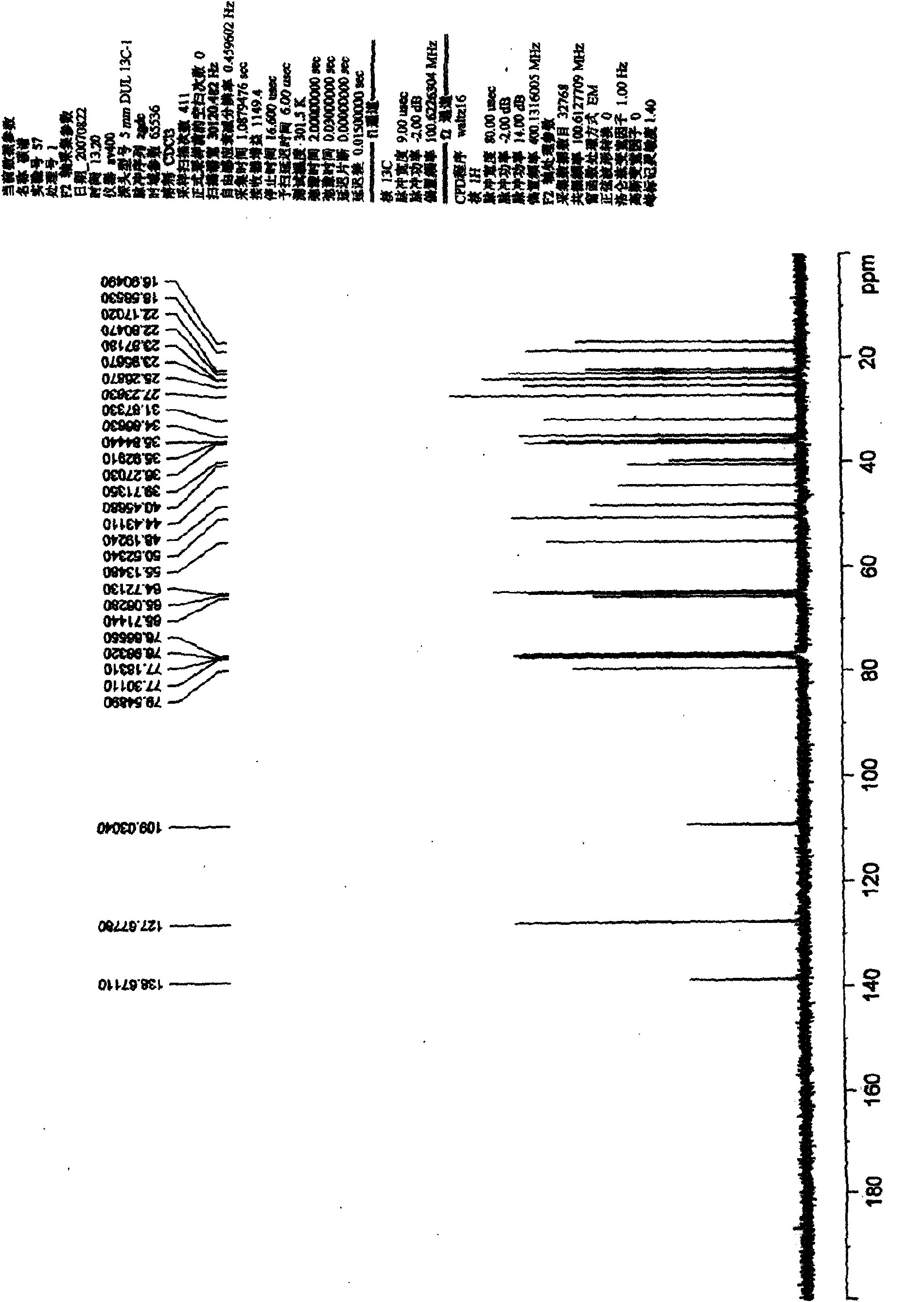
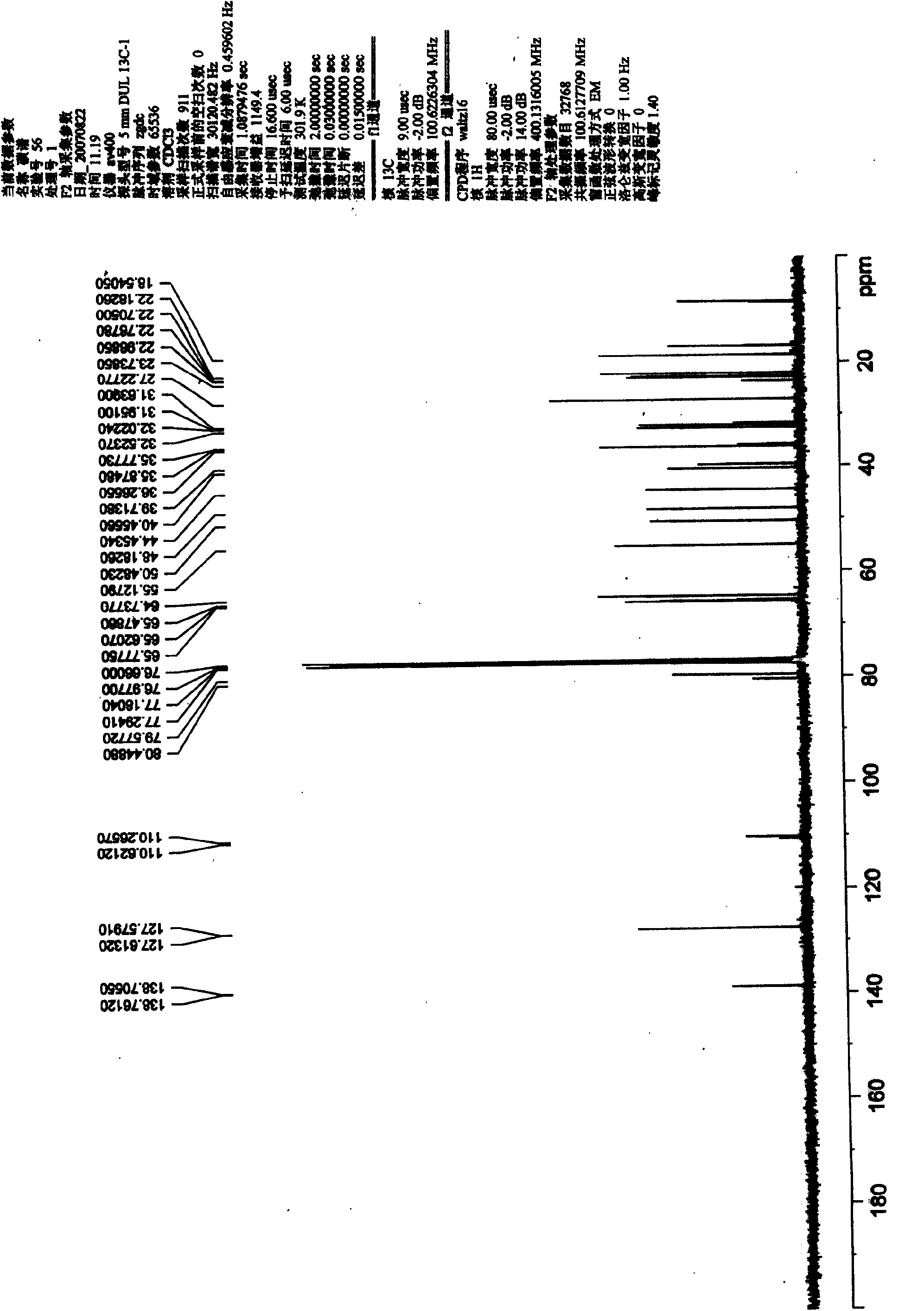
[0054] Preparation and Structure Identification of Chinonyl Alcohol Derivatives
[0055] 1. Dissolve 20 mg of chinonanol in 1 ml of acetone, add 20 μl of 2,2-dimethoxypropane and a catalytic amount of p-toluenesulfonic acid, and stir at room temperature for 20 minutes. A certain amount of water was added to the reaction mixture, and then extracted three times with ethyl acetate. The ethyl acetate phase was extracted with saturated sodium bicarbonate solution to remove p-toluenesulfonic acid present in the reaction as a catalyst, then extracted with saturated brine to remove water, and finally dried over anhydrous sodium sulfate overnight. The ethyl acetate phase dried overnight was concentrated, dissolved in dichloromethane and subjected to silica gel column chromatography, eluted with petroleum ether-acetone system (3:1) to obtain 15,16-isopropylidene chinonanol (compound 1) 18 mg, yield 90%.
[0056] Compound 1: C 23 h 28 o 5 (Mr. 378), white solid, mp. 198-200°C. MS (...
Embodiment 2
[0088] Experimental Study on Inhibition of the Release of Proinflammatory Factors TNF-α and IL-1β by Chinonyl Derivatives in Vitro
[0089] Experimental Materials:
[0090] 1. Cells: normal human peripheral blood mononuclear cells (PMBC)
[0091] Drugs to be tested: Chinonanol and its derivatives, separated and prepared by Shandong Target Drug Research Company.
[0092] 2. Positive control: dexamethasone (product of Sigma, USA)
[0093] 3. Reagents: Ficoll-Paque Plus (Amersham Bioscience); Endotoxin (LPS, Escherichia coli0111: B4, Sigma) and dexamethasone (DEX, CalBiochem); ELISA assay kit for TNF-α and IL-1β (Jingmei Biological engineering company); dimethyl sulfoxide (DMSO, Sigma); trypsin (Amresco); RPMI1640 medium, penicillin, streptomycin and fetal bovine serum (Gibco).
[0094] 4. Instrument: CKX31 inverted microscope (Olympus); constant temperature CO 2 Incubator (Thermo); 5810R desktop high-speed low-temperature centrifuge (Eppendorf); automatic microplate reader (...
Embodiment 3
[0111] Experimental Study on Inhibition of NO Release from RAW264.7 Cells by Chinonyl Alcohol Derivatives
[0112] Experimental Materials:
[0113] 1. Cells: mouse mononuclear macrophage cell line RAW264.7, purchased from Shanghai Cell Bank of Chinese Academy of Sciences (ATCC)
[0114] Drugs to be tested: Chinonanol and its derivatives, separated and prepared by Shandong Target Drug Research Company.
[0115] 2. Reagents: RPMI1640 medium, penicillin, streptomycin, and fetal bovine serum were purchased from Gibco; trypsin was purchased from Amresco; LPS (Escherichia coli 0111: B4) was purchased from Sigma. Other commonly used reagents were domestic analytical grade.
[0116] 3. Instrument: CKX31 inverted microscope (Olympus); constant temperature CO 2 Incubator (Thermo); 5810R desktop high-speed low-temperature centrifuge (Eppendorf); automatic microplate reader (Biotek).
[0117] experimental method:
[0118] RAW264.7 cells with 10% fetal bovine serum, 100 μg mL -1 Stre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com