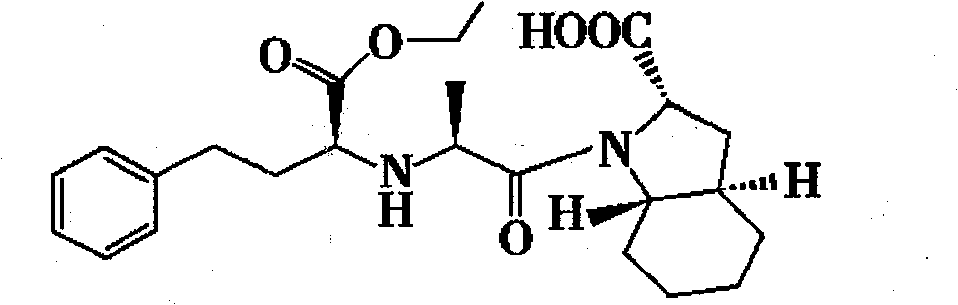
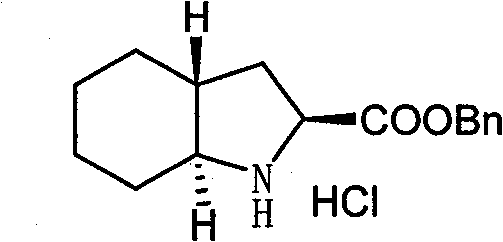
Method for preparing trandolapril intermediate
A trandolapril and intermediate technology, applied in the field of pharmaceutical intermediates, can solve the problems of difficulty in obtaining, long process route, difficult control of selective hydrogenation, etc., achieves good stereoselectivity, overcomes flammability and explosion, and shortens reaction steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
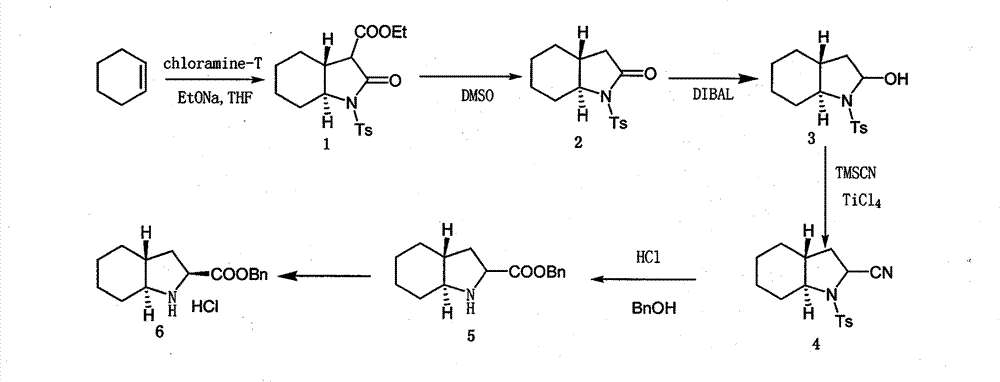
[0033] A preparation method of trandolapril intermediate, the method may further comprise the steps:
[0034] (1) Synthesis of (3aR, 7aS)-octahydro-2-oxo-1-(4-methylbenzenesulfonyl) ethyl 1-H-indole-3-carboxylate (hereinafter referred to as compound 1)
[0035] Chloramine-T (500g, 1.775mol), cyclohexene (164g, 2.0mol), I 2 (28.2g) was added into 1000ml tetrahydrofuran, reacted at room temperature for 24 hours, and placed for later use. At room temperature, add 320 g of diethyl malonate into a solution of 170 g of sodium ethoxide in THF (788 ml), raise the temperature and reflux for 45 minutes, slowly add the previous reaction solution dropwise within 4 hours, reflux for 15 hours, cool down to room temperature, and depressurize Evaporate the solvent, stir and dissolve with 1200ml ethyl acetate and 800ml water, adjust the pH to 6-7 with concentrated hydrochloric acid, separate the layers, extract the aqueous layer with ethyl acetate (400ml×3), combine the organic layers, wash wit...
Embodiment 2
[0047] A preparation method of trandolapril intermediate, the method may further comprise the steps:
[0048] (1) Synthesis of compound 1
[0049] Chloramine-T (500g, 1.775mol), cyclohexene (145g, 1.775mol), I 2 (33g) was added in 533ml tetrahydrofuran, reacted at room temperature for 24 hours, and placed for later use; at room temperature, 284g (1.775mol) diethyl malonate was added in THF (600ml) solution of 239.7g (4.44mol) sodium methylate, Raise the temperature and reflux for 45 minutes, slowly add the previous reaction solution dropwise within 4 hours, reflux for 15 hours, cool down to room temperature, evaporate the solvent under reduced pressure, stir and dissolve with 1000ml ethyl acetate and 600ml water, adjust the pH to 6 with concentrated hydrochloric acid ~7, separate layers, extract the aqueous layer with ethyl acetate (500ml×3), combine the organic layers, dry with anhydrous sodium sulfate, and evaporate the solvent under reduced pressure to obtain 551.3g of com...
Embodiment 3
[0061] A preparation method of trandolapril intermediate, the method may further comprise the steps:
[0062] (1) Synthesis of Compound 1
[0063] Chloramine-T (500g, 1.775mol), cyclohexene (218g, 2.663mol), I 2 (25g) was added in 1420ml tetrahydrofuran, reacted at room temperature for 24 hours, and placed for later use; at room temperature, 426g (2.663mol) diethyl malonate was added in THF (900ml) solution of 181.2g (2.663mol) sodium ethylate, Raise the temperature and reflux for 45 minutes, slowly add the previous reaction solution dropwise within 4 hours, reflux for 15 hours, cool down to room temperature, evaporate the solvent under reduced pressure, stir and dissolve with 1500ml ethyl acetate and 900ml water, adjust the pH to 6 with concentrated hydrochloric acid ~7, separate layers, extract the aqueous layer with ethyl acetate (300ml×3), combine the organic layers, dry with anhydrous sodium sulfate, and evaporate the solvent under reduced pressure to obtain 570.7g of co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com