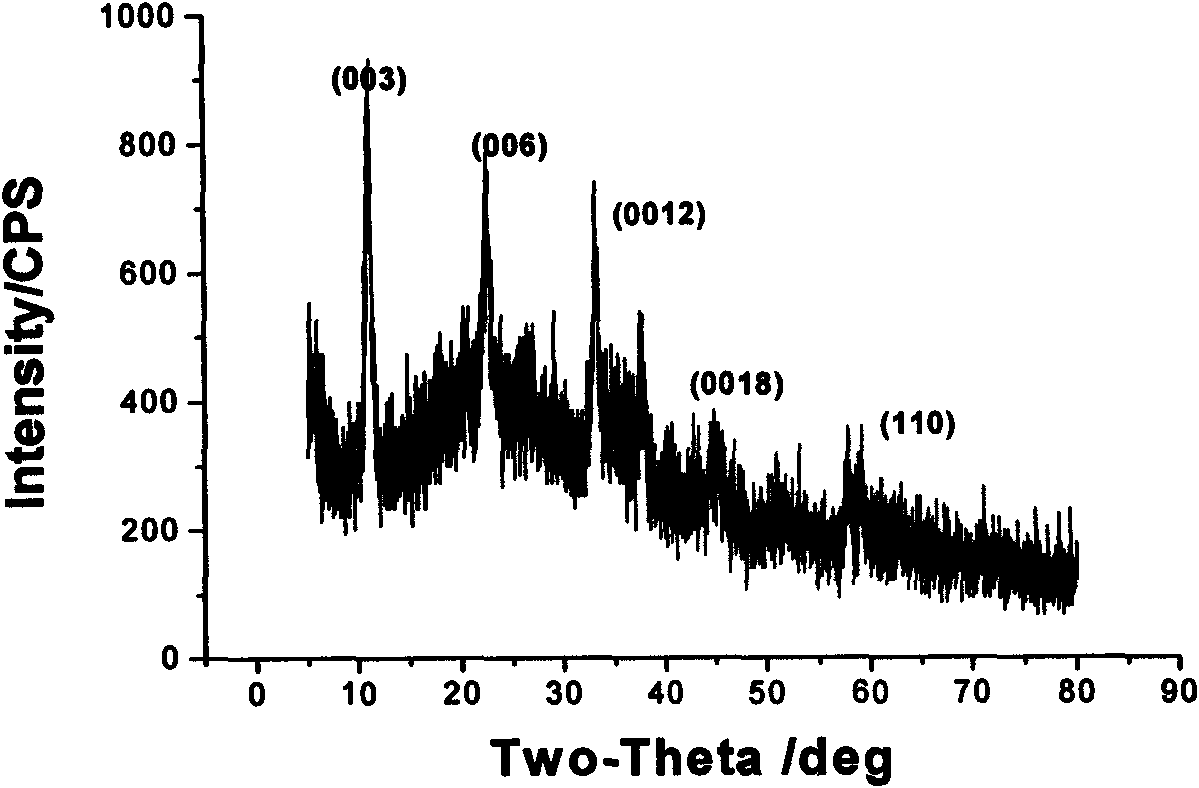
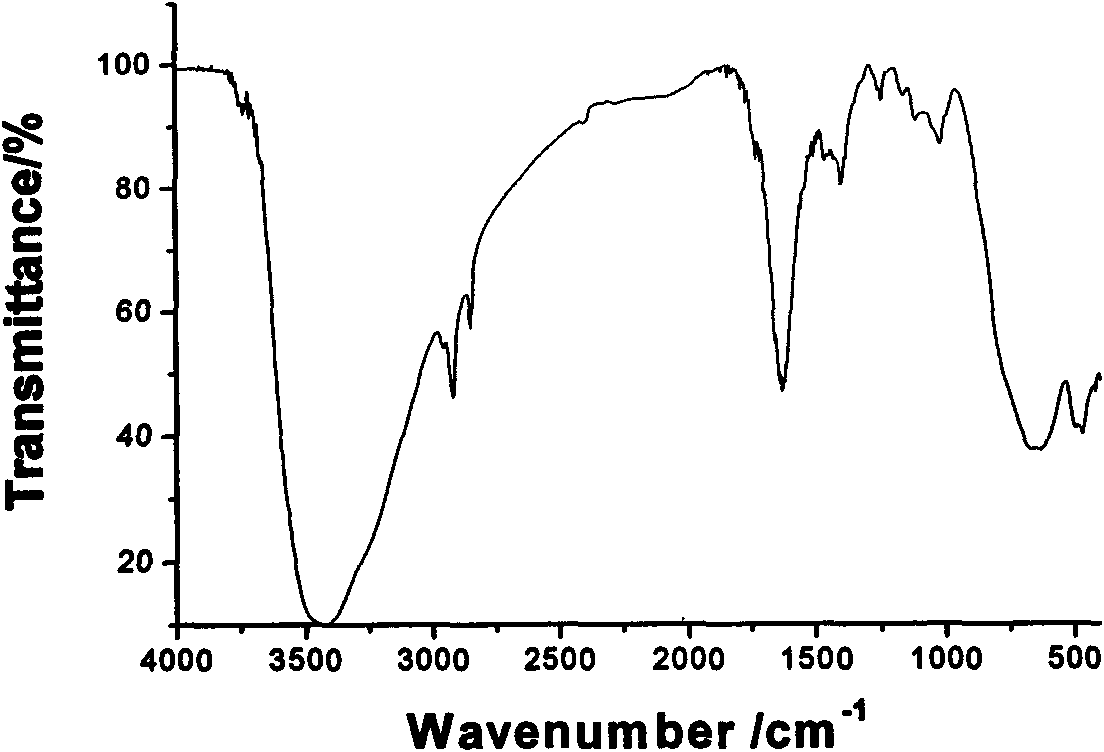
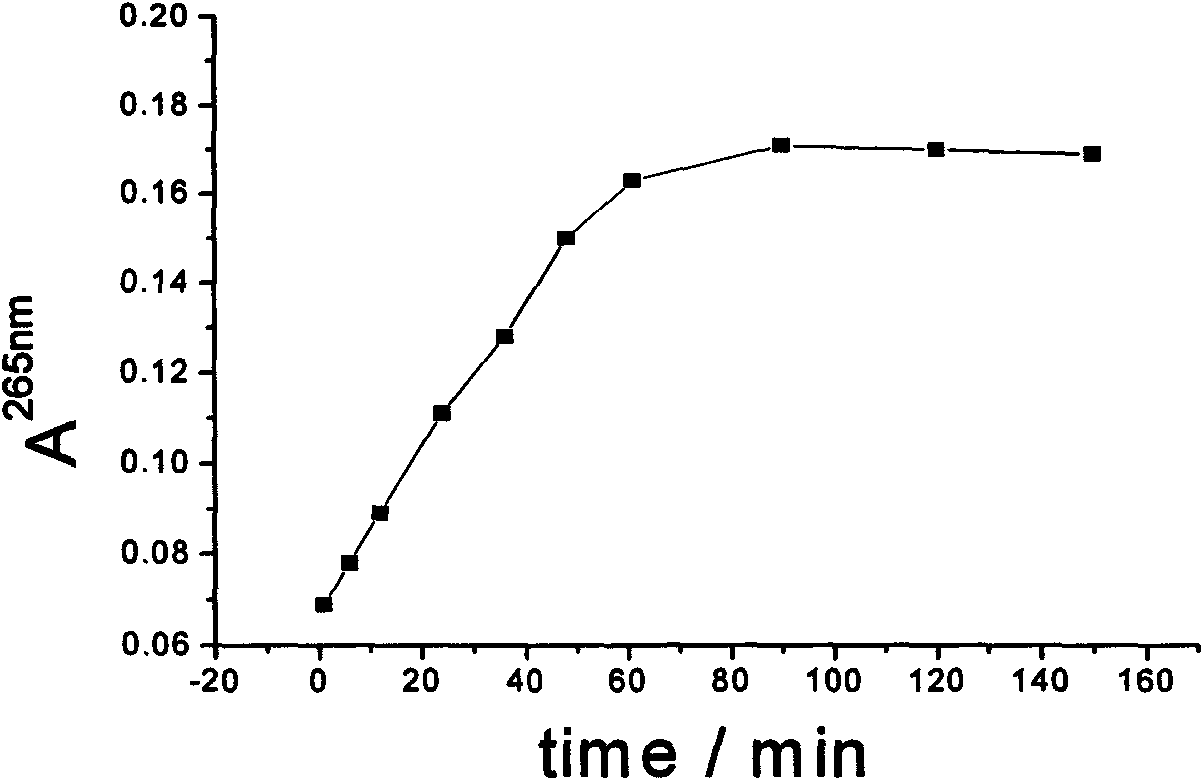
Method for synthesizing dextran-magnetic LDH-fluorouracil magnetic targeting sustained and controlled release tripolymer
A technology of fluorouracil and dextran, which is applied in the field of joint preparation of "nitrogen-protected co-precipitation intercalation-dextran compound-solvent conversion", which can solve the problems of unstable storage of magnetic LDH-Drugs, difficulty in slow-release and targeted fusion, and magnetic LDH is easy to oxidize and other problems, and achieves the effect of solving the problems of preparation and anti-oxidation protection, wide application range and high storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 7.5g FeCl 2 4H 2 O, 3.4 g FeCl 3 ·6H 2 O, 2.0g 5-Flu (fluorouracil), with 150mL H 2 O is dissolved under the conditions of 35-70°C, stirring speed 200rpm and nitrogen protection, and 2mol L is added dropwise over 15-30min. -1 NaOH solution to pH 6.0-8.0 at the end point of precipitation. After crystallizing the original slurry under the same conditions for 45-60 minutes, add 8.0-16.0 g of dextran to the reaction device, increase the stirring speed to 300 rpm, and react for 40-80 minutes at 35-70 ° C and nitrogen. Seal the reaction slurry and quickly transfer it to 500~1000mL of absolute ethanol that has been cryogenically treated at -10~-30°C for 2~4h, stir for 5~10min, then let it stand for 15~30min to complete the solvent conversion, and centrifuge (speed 5000rpm , 15min). Drying conditions: After the vacuum drying box is filled with nitrogen and emptied, transfer to the sample, set at 50-80°C, vacuum degree 0.085MPa, and dry for 24-48 hours.
[0026] The ferro...
Embodiment 2
[0028] 3.7g FeCl 2 4H 2 O, 1.7 g FeCl 3 ·6H 2 O with 150mL H 2 O is dissolved at 35-70°C, 200rpm and under nitrogen protection conditions, and 2mol L is driven dropwise by a constant flow of nitrogen -1 NaOH to the pH of the precipitation end point (6.0-8.0), maintain the reaction temperature of 35-70°C through the agitator and relay; crystallize the co-precipitation slurry under the same stirring speed, protective gas flow rate and 35-70°C for 45-60 minutes ; Then add 0.5-1.0g fluorouracil to the crystallization slurry, maintain the same conditions for ion exchange reaction for 1-4 hours, then add 4.0-8.0g dextran, increase the stirring speed to 300rpm, and perform compound reaction at 35-70°C and nitrogen protection conditions 40~80min. Seal the reaction slurry and quickly transfer it to 500~1000mL of absolute ethanol that has been cryogenically treated at -10~-30°C for 2~4 hours, complete the solvent conversion treatment under stirring conditions, and then centrifuge a...
Embodiment 3
[0030] Press Fe 2+ / Al 3+ Molar ratio 3 will 8mL 1.50mol L -1 FeCl 2 and 8mL 0.50mol·L -1 AlCl 3 The solution was driven into the synthesis device by high-purity nitrogen, and 0.5 g of fluorouracil was dissolved in the mixed salt solution under constant nitrogen protection and 200 rpm stirring conditions, and 2.0 mol L was added dropwise. -1 NaOH precipitant until the end point pH is 6.0-8.0, and maintain the reaction temperature of 35-70°C; the co-precipitation slurry is crystallized for 45-60 minutes under the same stirring speed, protective gas flow rate and 35-70°C; then add 4.0 to the synthesis device ~8.0g dextran, increase the stirring speed to 300rpm, compound reaction at 35~70℃ and nitrogen protection conditions for 40~80min; seal the reaction slurry, quickly transfer it to 500~1000mL, and undergo cryogenic treatment at -10~-30℃ for 2~ In 4 hours of absolute ethanol, stir to complete the solvent conversion treatment, let stand for about 15-30 minutes, and then ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com