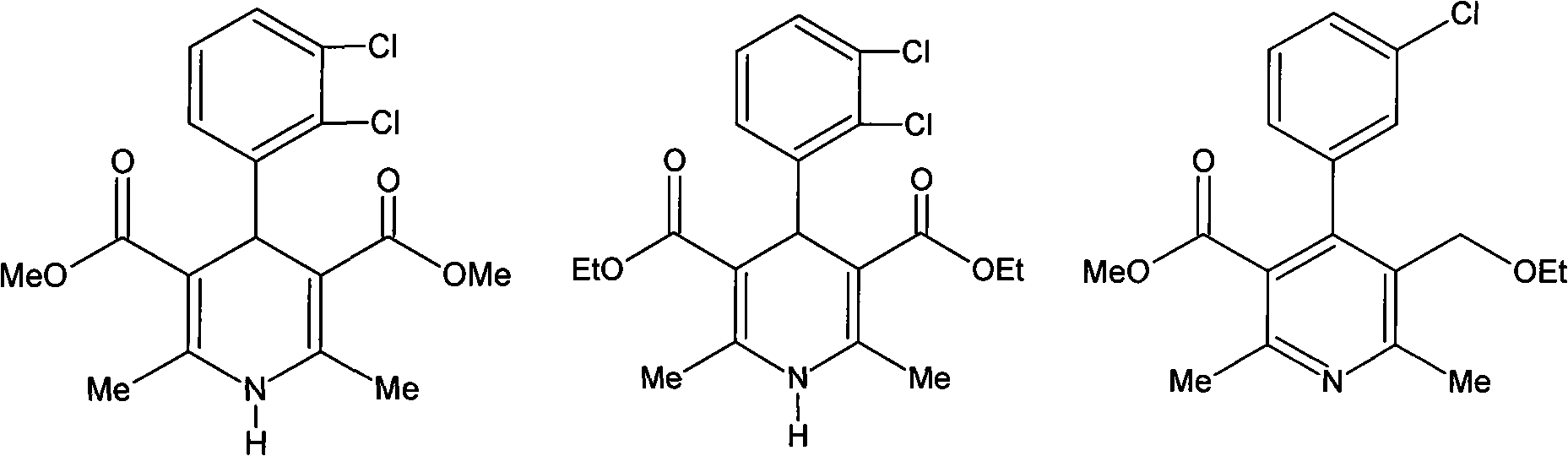
Preparation method of antihypertensive drug felodipine
A felodipine and anti-hypertensive technology, which is applied in the field of organic chemical synthesis, can solve the problems of lengthy and cumbersome post-processing operations, and achieve the effects of ideal purity, short reaction time and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0034] The preparation of example 1 Felodipine:
[0035] 1.1. Cyclosynthesis and addition:
[0036] Add 640ml of absolute ethanol, 77.5ml of absolute ethanol, and g (pure: 0.59 mol.) ethyl 3-aminocrotonate and 6.9 g D072 type resin. Nitrogen flow was introduced into the bottle under stirring, and then 138.2 g (0.5 mol of pure equivalent) of methyl 2,3-dichlorobenzylidene acetoacetate was added. After the addition was completed, the temperature of the water bath was raised to 65° C. to 70° C., and the reaction was continued for 6 hours. Then the temperature was raised to reflux state to continue the reaction for 2 hr (the end point of the reaction can be determined by TLC method). The reaction solution was cooled to 30°C, the catalyst was filtered off, and rinsed with the same solvent until clear. Combine the filtered and washing liquids, and add 400ml of cyclohexane when vacuum-concentrating on a water bath to about 1 / 3 of the original volume. After the addition is complet...
Embodiment 2
[0044] Embodiment 2. The preparation of felodipine:
[0045] 1. Cycloaddition
[0046] In this experiment, the specifications, consumption and reaction temperature, time, concentration, precipitation and drying conditions of each raw material and catalyzer are the same as example 1, only use isopropanol (640ml.) n-heptane to replace dehydrated alcohol and n-hexane in example 1 alkyl. According to the law, 177.9 g of off-white powdery felodipine crude product was obtained, with a purity of 98.9% and a yield of 91.6%.
[0047] 2. Felodipine crude product recrystallization
[0048] Method, condition and result are the same as example 1.
Embodiment 3
[0049] Embodiment 3. Felodipine crude product preparation (imitation US2004 / 0204604A method):
[0050] 124.4 g (pure: 0.45 mol) of methyl 2,3-dichlorobenzylidene acetoacetate was mixed with 590 ml of isopropanol, and then 70.6 g (pure: 0.54 mol) of ethyl 3-aminocrotonate was added. The reaction mixture was refluxed on a water bath for 12 hours. The reaction solution was placed on a water bath to evaporate isopropanol under reduced pressure, and 390 ml of n-heptane was added with stirring to precipitate felodipine. After standing at room temperature for 1 hour, it was filtered. The filter cake was washed three times with n-heptane, and vacuum-dried to dryness below 50° C. to obtain 147.9 g of yellow hard lump felodipine crude product. The purity is 96.3%, and the pure yield is 82.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com