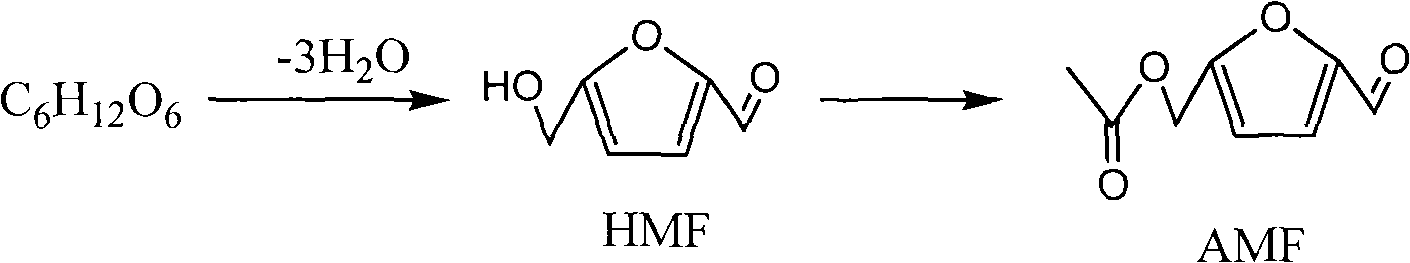
Method for preparing 5-acetoxymethyl furfural with carbohydrate
A technology of acetoxymethylfurfural and carbohydrates, which is applied in the field of preparing 5-acetoxymethylfurfural, can solve the problems that the catalyst cannot be recycled and reused, the cost is high, and the yield is low, and it has a good industrial application prospect and no equipment Corrosion, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Soak 301 type weak base anion exchange resin with 10% NaCl solution for 8h, then wash with distilled water until AgNO 3 The solution was detected until there was no white precipitate, and then exchanged with ammonium acetate solution with a mass fraction of 7%, and then washed with distilled water until AgNO 3 The solution is tested until there is no white precipitate, then suction-filtered and dried to obtain an acetate-exchanged weak base anion-exchange resin.
[0015] Add 0.09g fructose (0.5mmol) and 1mL dimethyl sulfoxide to a single-necked round bottom flask, then add 0.09g acidic cation exchange resin, connect the condenser and place it in an oil bath, and raise the temperature of the oil bath to 100 ℃, constant temperature, stirring, and stop the reaction after 6 hours of reaction. The reaction system was cooled to room temperature with water, and the catalyst was separated by centrifugation. High performance liquid chromatography detection showed that the yiel...
Embodiment 2
[0017] Add 0.09g fructose (0.5mmol) and 1mL dimethyl sulfoxide to a single-necked round bottom flask, then add 0.09g acidic cation exchange resin, connect the condenser and place it in an oil bath, and raise the temperature of the oil bath to 100 ℃, constant temperature, stirring, and stop the reaction after 6 hours of reaction. The reaction system was cooled to room temperature with water, and the catalyst was separated by centrifugation. High performance liquid chromatography detection showed that the yield of HMF was 93%. 4A molecular sieve is used to remove the water generated by the dehydration reaction, and the 4A molecular sieve is separated by filtration. Acetic anhydride was added according to the molar ratio of HMF and acetic anhydride being 1:3, and the reaction was stopped after reacting at 70° C. for 1 hour. The reaction system was cooled to room temperature with water. High-performance liquid chromatography detection showed that the yield of AMF was 71%, calcu...
Embodiment 3
[0019] Add 0.09g fructose (0.5mmol) and 1mL dimethyl sulfoxide to a single-necked round bottom flask, then add 0.09g acidic cation exchange resin, connect the condenser and place it in an oil bath, and raise the temperature of the oil bath to 100 ℃, constant temperature, stirring, and stop the reaction after 6 hours of reaction. The reaction system was cooled to room temperature with water, and the catalyst was separated by centrifugation. High performance liquid chromatography detection showed that the yield of HMF was 93%. 4A molecular sieve is used to remove the water generated by the dehydration reaction, and the 4A molecular sieve is separated by filtration. Add 0.05 g of acetate-exchanged weak base anion exchange resin prepared according to Example 1, and add acetic anhydride according to the molar ratio of HMF to acetic anhydride of 1:3, and stop the reaction after reacting at 70° C. for 1 hour. The reaction system was cooled to room temperature with water, and the ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com