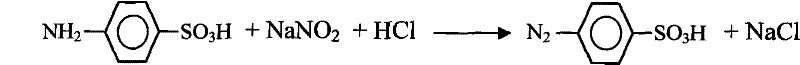Carbon-based solid acid catalyst and preparation method thereof
A carbon-based solid acid and catalyst technology, which is used in catalyst activation/preparation, carboxylate preparation, chemical instruments and methods, etc., can solve the problems of weak acidity and high cost, and achieve low production cost, low price and high mechanical strength. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 0.075mol p-aminobenzenesulfonic acid and 187ml, 1mol / l hydrochloric acid into the three-necked flask to form a reaction system, turn on the electromagnetic stirring, control the temperature of the reaction system at 4°C with an ice-water bath, and use a separatory funnel in the reaction system Slowly add 80ml, 1mol / l sodium nitrite solution, stir and react for 30min to obtain a white precipitate, diazonium benzenesulfonate, wash the diazonium benzenesulfonate with distilled water, filter and set aside.
[0024] The chemical reaction formula of the reaction system is as follows:
[0025]
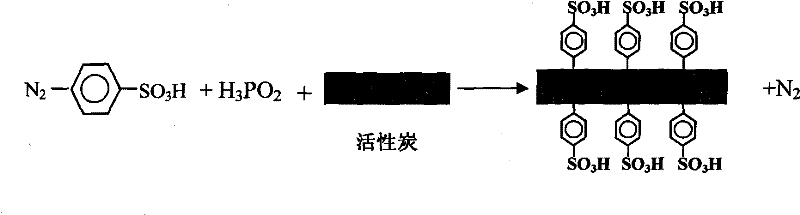
[0026] Then, 1.5 g of activated carbon, 100 ml of absolute ethanol, 100 ml of distilled water, and 400 ml of 50 wt % hypophosphorous acid and the prepared diazonium benzenesulfonate were added to the three-necked flask for 90 minutes of reaction.
[0027] The chemical reaction formula of the reduction reaction is as follows:
[0028]
[0029] The activated carbon was washed ...
Embodiment 2
[0032] Add 0.075mol of p-aminobenzenesulfonic acid and 187ml of 1mol / l hydrochloric acid into the three-necked flask to form a reaction system, turn on the electromagnetic stirring, control the temperature of the reaction system at 2°C with an ice-water bath, and use a separatory funnel in the reaction system Slowly add 80ml, 1mol / l sodium nitrite solution, stir and react for 60min to obtain a white precipitate, diazonium benzenesulfonate, wash the diazonium benzenesulfonate with distilled water, filter and set aside. The chemical reaction formula of reaction system is the same as embodiment 1.
[0033] Then, 1.5 g of activated carbon, 100 ml of absolute ethanol, 100 ml of distilled water, and 400 ml of 50 wt % hypophosphorous acid and the prepared diazonium benzenesulfonate were added to the three-necked flask for 90 minutes of reaction. The activated carbon was washed with distilled water and acetone, and dried in an oven at 120°C for 12 hours to obtain a carbon-based solid ...
Embodiment 3
[0036] Add 0.075mol p-aminobenzenesulfonic acid and 187ml, 0.5mol / l sulfuric acid into the three-necked flask to form a reaction system, turn on the electromagnetic stirring, control the temperature of the reaction system at 2°C with an ice-water bath, and use a separatory funnel in the reaction system Slowly add 80ml, 1mol / l potassium nitrite solution, and stir for 90min to obtain a white precipitate, diazonium benzenesulfonate. Wash the diazonium benzenesulfonate with distilled water and filter for later use. The chemical reaction formula of reaction system is the same as embodiment 1.
[0037] Then, 2.0 g of activated carbon, 100 ml of absolute ethanol, 100 ml of distilled water, 400 ml of 50 wt % hypophosphorous acid and the prepared diazonium benzenesulfonate were added to the three-necked flask, and reacted for 120 min. Wash with distilled water and acetone, and dry in an oven at 120° C. for 12 hours to obtain a carbon-based solid acid catalyst. The chemical reaction fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com