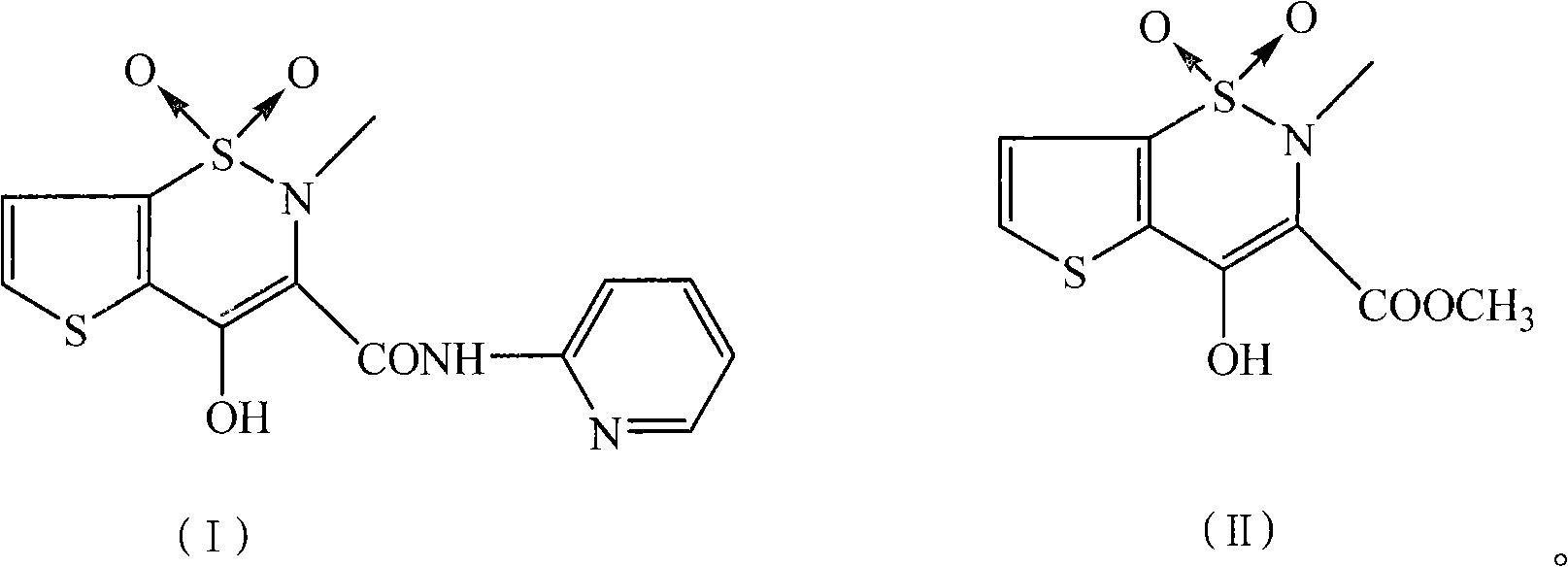
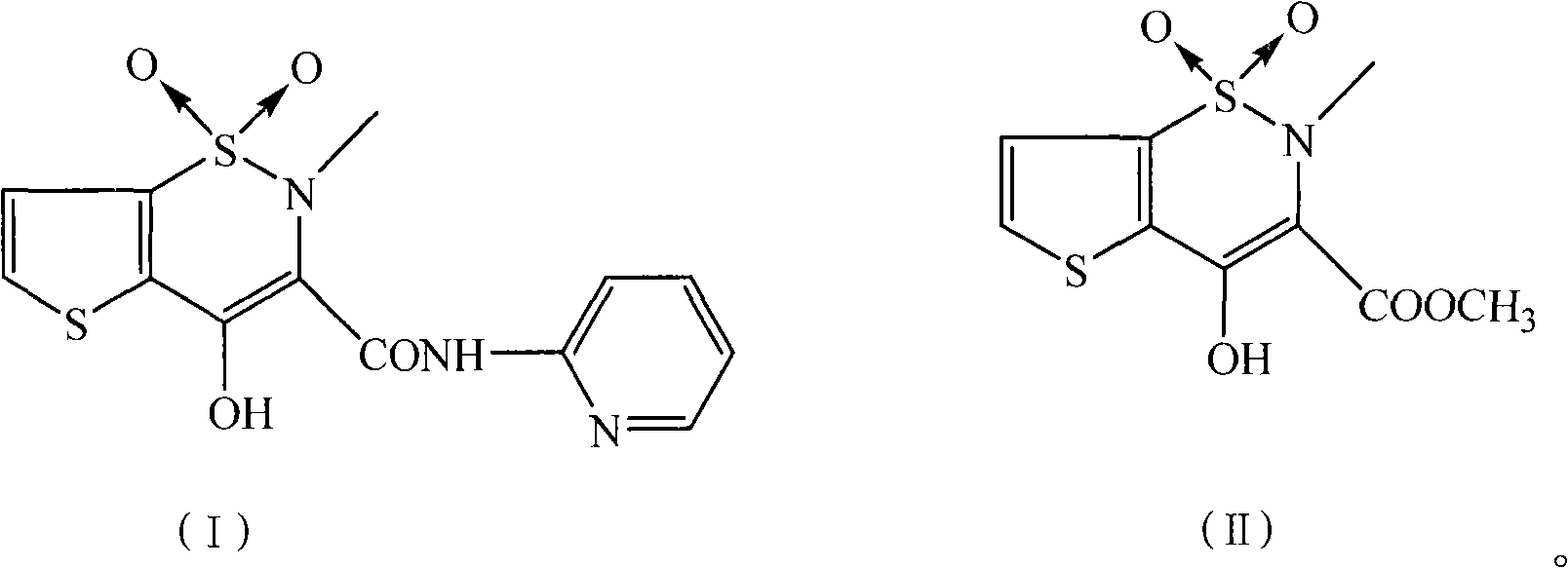
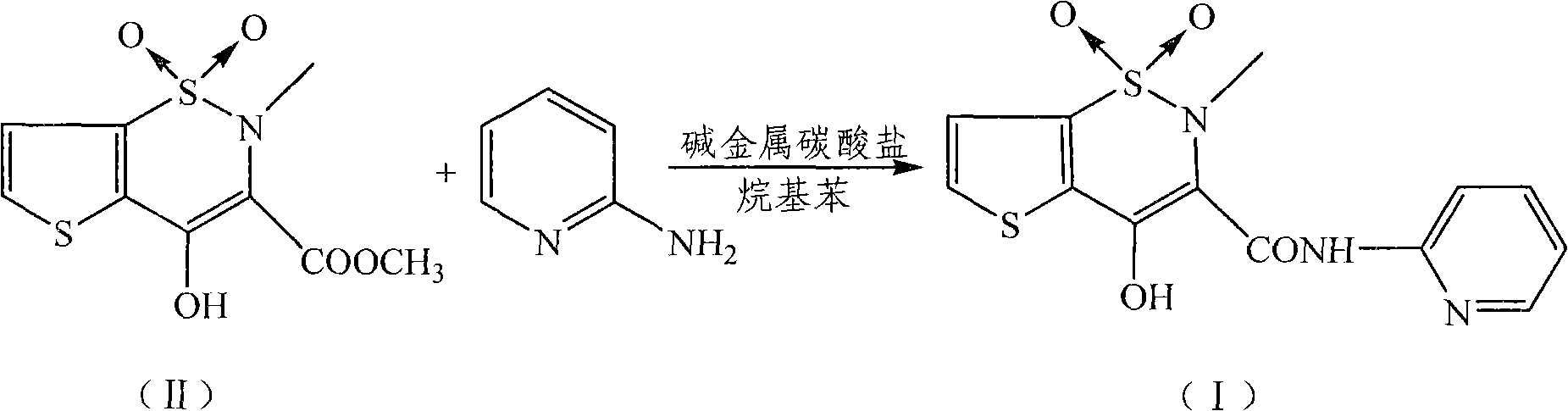
Preparation method of tenoxicam
A technology of tenoxicam and tenoxicam methyl compound, which is applied in the field of preparation of tenoxicam, can solve the problems of difficult separation and purification of impurities, low product purity, poor product color and luster, and achieves complete reaction and reaction temperature. The effect of reducing and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 4.5 g of tenoxicam first compound (16.3 mmol), 1.8 g of 2-aminopyridine (19.1 mmol), 3.0 g of potassium carbonate (21.7 mmol) and 300 ml of xylene in a 500 ml three-necked flask, and heat to Reflux and stir the reaction for 16 hours, then cool, cool and crystallize at 10°C for 5 hours, and filter. After the filter cake is dried, add it to a mixed solution of 40 milliliters of methanol, 10 milliliters of water and 1 gram of sodium hydroxide, heat to dissolve, add 0.54 grams of activated carbon for decolorization, filter, and the filtrate is adjusted to pH=2.5 with 20% hydrochloric acid, and static at 5 ° C. After standing for 10 hours, it was filtered and dried to obtain 4.80 g of a yellow solid with a yield of 87.0% and a melting point of 207-208.5°C.
Embodiment 2
[0027] In a 500 ml three-necked flask, add 4.5 g of tenoxicam first compound (16.3 mmol), 2.0 g of 2-aminopyridine (21.3 mmol), 2.5 g of sodium carbonate (23.6 mmol) and 150 ml of xylene, and heat to After reflux and stirring for 13 hours, the crystallization was cooled at 10°C for 5 hours and filtered. After the filter cake is dried, add it to a mixed solution of 28 milliliters of methanol, 9 milliliters of water and 1.3 grams of sodium hydroxide, heat to dissolve, add 0.45 grams of activated carbon for decolorization, filter, and the filtrate is adjusted to pH=3.0 with 20% hydrochloric acid, and static at 5 ° C. After standing for 5 hours, it was filtered and dried under vacuum to obtain 4.91 g of a yellow solid with a yield of 89.0% and a melting point of 207-209°C.
Embodiment 3
[0029] In 500 milliliters of there-necked flasks, add 4.5 gram tenoxicam first compounds (16.3 mmol), 1.6 gram 2-aminopyridine (17 mmol), 4.5 gram sodium carbonate (42.5 millimoles) and 150 milliliters of xylenes, stir, After reacting at 130°C for 12 hours, cooling to crystallize at 5°C, and filtering. After the filter cake is dried, add it to a mixed solution of 40 milliliters of methanol, 9 milliliters of water and 0.5 grams of sodium hydroxide, heat to dissolve, add 0.45 grams of activated carbon for decolorization, filter, and the filtrate is adjusted to pH=3.0 with 20% hydrochloric acid, and static at 10 ° C. After standing for 5 hours, it was filtered and dried in vacuo to obtain 4.98 g of a yellow solid with a yield of 90.3% and a melting point of 207.3-209°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com