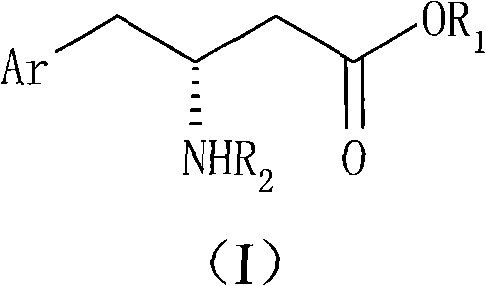
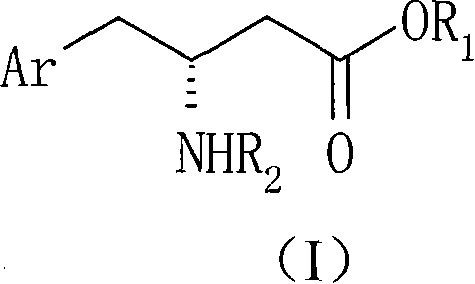
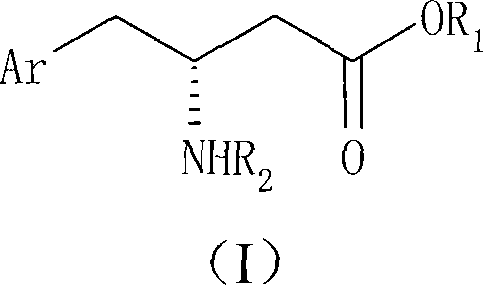
Method for preparing R-beta-aminobenzene butyric acid derivative
一种氨基苯丁酸、氨基苯丁酸酯的技术,应用在R-β-氨基苯丁酸衍生物领域,能够解决手性还原催化剂不易制取、手性还原工艺操作过程繁琐、结果不能让人满意等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0044] 114g (0.60mol) of 2,4,5-trifluorophenylacetic acid, add THF 600ml, slowly add N, N-carbonyldiimidazole 107g (0.66mol) under stirring (a large amount of solids will appear after adding a part, and then with further After the addition, the temperature was raised to 50° C., 95.1 g (0.66 mol) of McBurney’s acid was added, and the reaction was kept for 3 hours. Concentrate to remove THF, add 600ml of water and 800ml of dichloromethane, adjust the pH of the water layer to 2, separate the organic phase and wash with 0.1N HCl, then wash with 600ml of water, dry and concentrate to obtain 182g of solid condensate 2,2-dimethyl-5 -[2-(2,4,5-trifluorophenyl)-acetyl]-[1,3]dioxolane-4,6-dione (white solid powder can be obtained by recrystallization of ethyl acetate), Melting point: 101.5-103.5°C, yield 96%.
Embodiment 1
[0046] Add 60 g (0.190 mol) of the condensate of Preparation Example 1 to 600 ml of ethanol, stir and heat to 70 ° C for 3 hours to react, and the ethanol solution of ethyl 2,4,5-trifluorophenylacetoacetate (II) is obtained at this time 70g (1.11mol) of ammonium formate was added to the reaction solution, refluxed for 3 hours, slightly cooled to about 40°C, 15g (0.239mol) of sodium cyanoborohydride was slowly added, and then refluxed for 2 hours. After cooling, concentrate to remove ethanol, then add water, adjust the pH value to 9, extract with dichloromethane, wash with a small amount of water, dry and concentrate to obtain 45 g of brown oily ethyl β-amino-2,4,5-trifluorophenylbutyrate. The rate is 90.5%.
Embodiment 2
[0048] β-Amino-2,4,5-trifluorophenylbutyric acid ethyl ester racemate 5.18g (20mmol), dissolved in methanol 60ml, D-DTTA 3.86g (10mmol) was added under stirring, a large amount of white solid precipitated out quickly , heated to reflux for 1 to 2 hours (the solid cannot be completely dissolved), then cooled to below 10°C, filtered the precipitated solid, washed with a small amount of methanol, added methanol for recrystallization twice, and dried to obtain 3.37g of white solid powder, melting point: 187.0~ 188.0°C, [a] D 25 =+96.7° (C1, 0.1M NaOH), 3.0 g of the white solid was taken and dissociated to obtain 1.20 g of R-β-amino-2,4,5-trifluorophenylbutyric acid ethyl ester (Ib) with optical purity above 99.7%. The yield of one resolution is 52.2%.
[0049] Combine the mother liquors obtained from the above resolution and two crystallization processes, concentrate to dryness and then free with a saturated solution of sodium bicarbonate, and extract with chloroform to obtain 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com