Immortalized human liver cell line, preparation method and application thereof
A liver cell and immortalization technology, applied in the field of biology, can solve problems such as liver cell function defects, immune rejection, virus infection, etc., and achieve good application prospects, avoid immune reactions, and prolong survival time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The establishment of embodiment 1 immortalized human liver cell line (IHH):
[0046] Acquisition of Primary Isolated Human Hepatocytes: Isolation of Hepatocytes from Hepatectomy Surgical Specimens by Collagenase Perfusion (Nguyen TH, Oberholzer J, Birraux J, et al. Highly efficient Lentiviral vector-mediated transduction of nondividing, fully reimplantable primary hepatocytes. Molecular therapy, 2002, 6(2): 199-209).
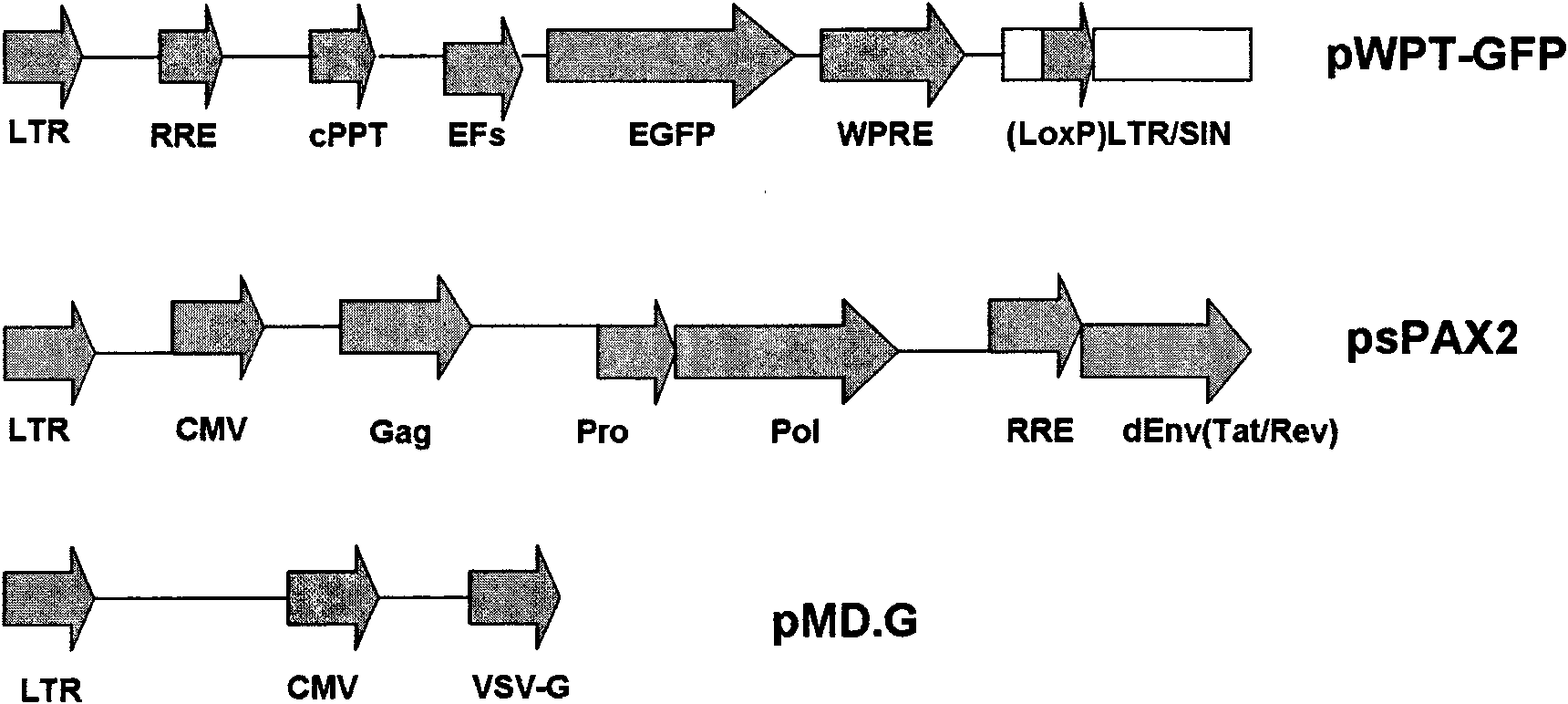
[0047] Vectors and plasmids: eg figure 1 As shown, the vector used included three plasmids: the pWPT-GFP gene transfer vector, containing the enhanced GFP gene (EGFP) driven by the CMV promoter; the viral packaging plasmid psPAX2, encoding the Gag and Pol precursors of HIV-1, and Regulatory proteins Tat and Rev; capsid-encoding plasmid pMD.G, which expresses the viral packaging protein VSV G. The plasmid Plox-Ttag-iresTK carrying the SV40 T antigen gene and the plasmid pBabe-hygro-hTert carrying the hTERT gene. (Vector plasmids were purchased from Addg...
Embodiment 2
[0059] The functional characteristic of embodiment 2 IHH:
[0060] To evaluate the functional properties of the immortalized human hepatocyte cell line obtained in Example 1, firstly, the RT-PCR method was used to detect the expression of adult hepatocyte specific molecular mRNA by IHH. Collect IHH cells, use Qiagen's RNA extraction kit to extract total RNA, then take 2ug of total RNA, and use Qiagen's reverse transcription kit to perform reverse transcription. Then use the Taq enzyme of Promega Company to carry out PCR amplification, wherein the primers used are synthesized by Shanghai Sangon Bioengineering Co., Ltd., and the specific primer sequences of each gene are as follows in Table 1:
[0061] Table 1. PCR amplification primer sequences
[0062]
[0063] The amplification conditions were as follows: initial denaturation temperature of 94°C for 2min, cycle denaturation temperature of 94°C for 30s, annealing temperature of 55°C for 30s, extension temperature of 72°C f...
Embodiment 3
[0066] Safety evaluation of embodiment 3 IHH:
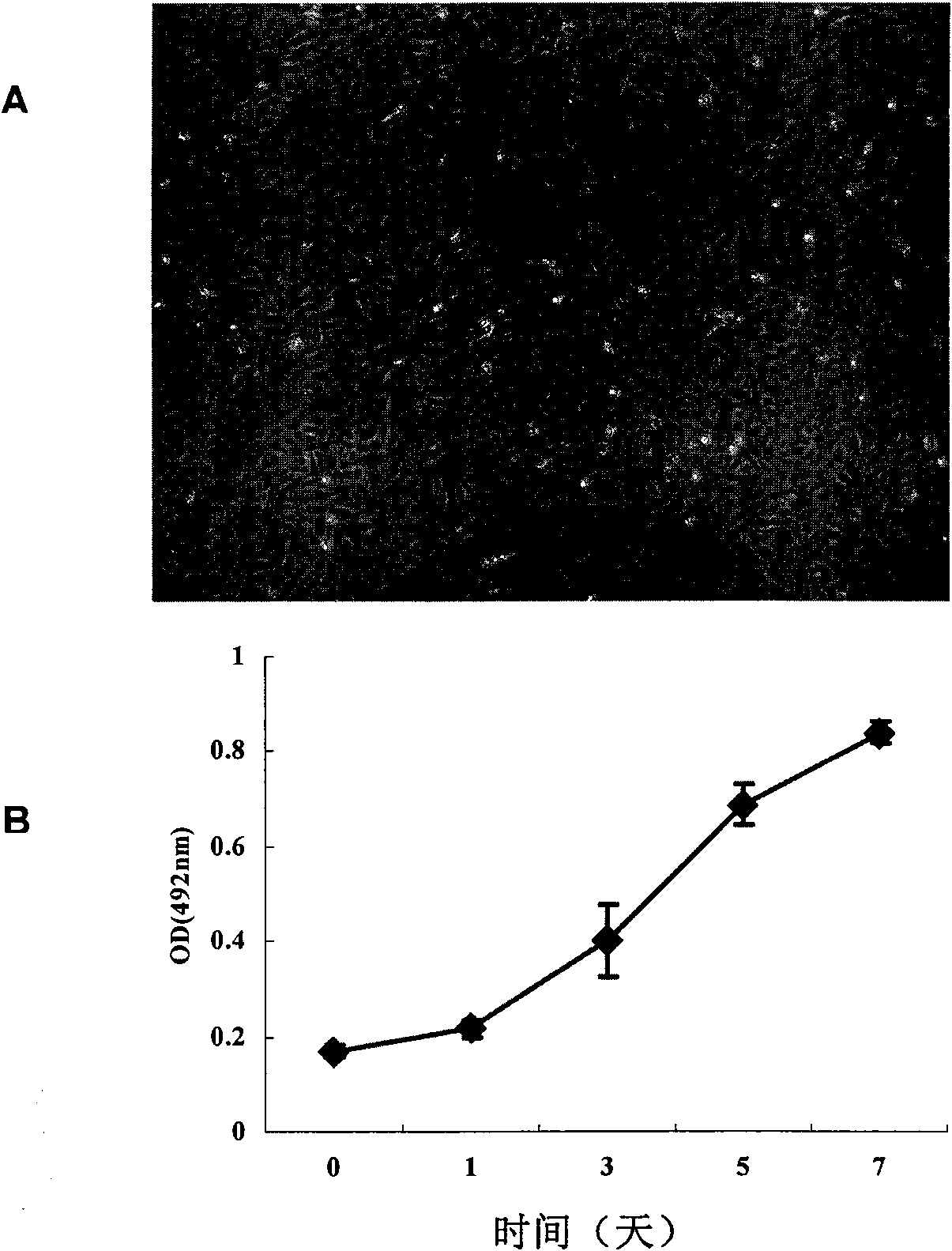
[0067] Get the IHH cell that embodiment 1 of logarithmic growth phase obtains, with Hank's solution (137.93mM NaCl, 5.33mMKCl, 4.17mM NaHCO , 1.26mM CaCl , 0.493mM MgCl , 0.441mM KHPO , 0.338mMNaHPO , 5.56mM D-glucose) After washing twice, resuspend in DMEM / F12 basal medium, inoculate the cells in subcutaneous and liver of 5 BALB / c nude mice, inoculate 5×10 6 cells. Another group of nude mice was simultaneously inoculated with liver cancer cell BEL-7402 subcutaneously and in the same part of the liver as a positive control, and each point was inoculated with 5×10 6 cells as a positive control. Continuous observation for 6 months after inoculation showed that there was no tumor formation in the subcutaneous and liver inoculation sites of the nude mice, while nude mice inoculated with the same number of liver cancer cells BEL-7402 at the same site had tumor formation in the subcutaneous and liver one month after transplantation. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com