Metallic oxide/carbon fiber composite material and preparation method and applications thereof
A composite material and carbon fiber technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, chemical/physical processes, etc. Large and special shape carbon fibers are difficult to prepare and control, so as to achieve the effect of increasing catalytic activity and increasing current density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
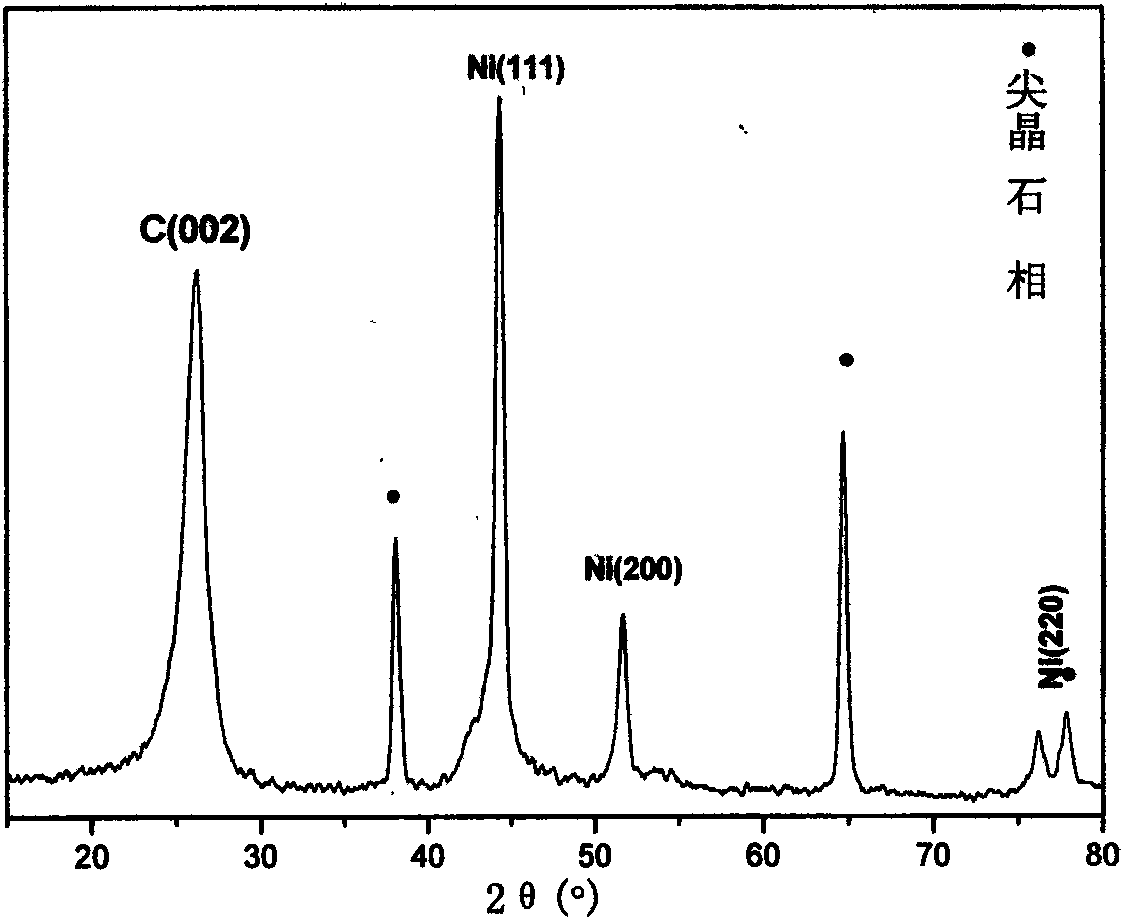
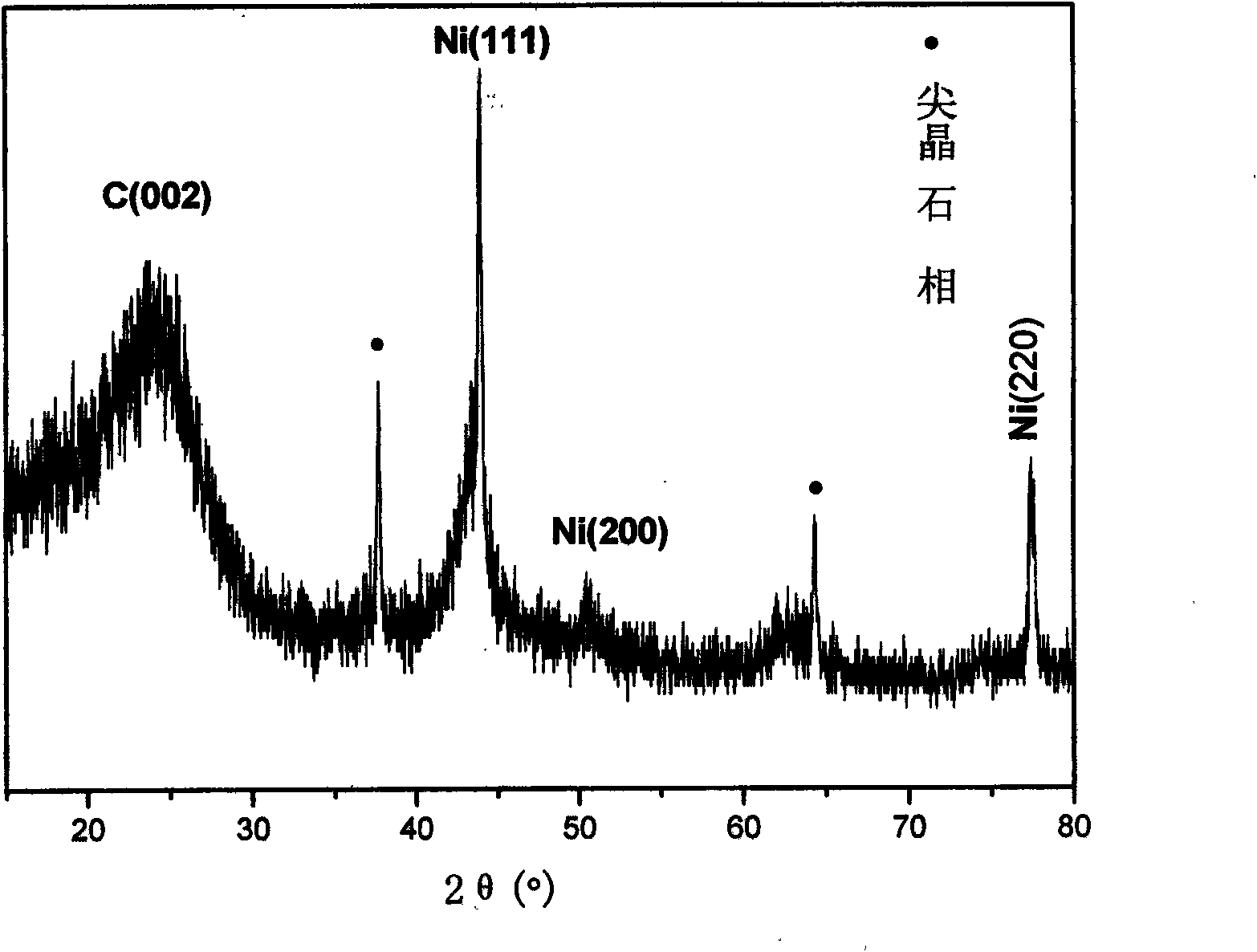
Image
Examples
Embodiment 1
[0025] With 80ml deionized water and a certain proportion of Ni(NO 3 ) 3 ·6H 2 O and Al(NO 3 ) 3 ·7H 2 O is prepared into a salt solution and then added appropriate urea to make a mixed solution, in which Ni 2+ The molar concentration of Al is 0.6mol / L, Al 3+ The molar concentration of urea is 0.3mol / L, and the molar concentration ratio of urea and all metal cations is 3.3. Put it into the liner of the polytetrafluoroethylene resin of 100ml, seal in the autoclave. Put it in an oven at 150°C for 24 hours for crystallization, and finally take out the liner. After the reaction was completed, it was suction filtered, washed twice with deionized water, and dried at 70° C. for 15 hours.
[0026] Spread 30 mg of the LDH precursor prepared above on a porcelain boat, and then put it into a horizontal quartz tube located in a tubular heating furnace, feed nitrogen gas with a flow rate of 60 ml / min, and raise the temperature to 700 °C at a rate of 5 °C / min. After 30min at ℃, ace...
Embodiment 2
[0030] With 80ml deionized water and a certain proportion of Cu(NO 3 ) 2 ·6H 2 O, Mg(NO 3 ) 3 ·6H 2 O and Fe(NO 3 ) 3 9H 2 O is prepared into a salt solution and then added appropriate urea to make a mixed solution, in which Cu 2+ The molar concentration is 0.8mol / L, Mg 2+ The molar concentration of Fe is 0.4mol / L, Fe 3+ The molar concentration of urea is 0.4mol / L, and the molar concentration ratio of urea and all metal cations is 6. Put it into the liner of the polytetrafluoroethylene resin of 100ml, seal in the autoclave. Put it into an oven at 100°C for crystallization for 15 hours, and finally take out the liner. After the reaction was completed, it was suction filtered, washed twice with deionized water, and dried at 70° C. for 15 hours.
[0031] Spread 30 mg of the LDH precursor prepared above on a porcelain boat, and then put it into a horizontal quartz tube located in a tubular heating furnace, feed nitrogen gas with a flow rate of 70 ml / min, and raise the ...
Embodiment 3
[0035] With 80ml deionized water and a certain proportion of Ni(NO 3 ) 3 ·6H 2 O, Cu(NO 3 ) 2 ·6H 2 O, Mg(NO 3 ) 2 ·6H 2 O, Al(NO 3 ) 3 ·7H 2 O is prepared into a salt solution and then added appropriate urea to make a mixed solution, in which Ni 2+ The molar concentration is 0.4mol / L, Cu 2+ The molar concentration is 0.4mol / L, Mg 2+ The total molar concentration of Al is 0.4mol / L, Al 3+ The molar concentration of urea is 0.3mol / L, and the molar concentration ratio of urea and all metal cations is 5. Put it into the liner of the polytetrafluoroethylene resin of 100ml, put into the autoclave and seal. Put it into an oven at 120°C for crystallization for 20 hours, and finally take out the liner. After the reaction was completed, it was suction filtered, washed twice with deionized water, and dried at 70° C. for 15 hours.
[0036] Spread 50 mg of the LDH precursor prepared above on a porcelain boat, and then put it into a horizontal quartz tube located in a tubula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com