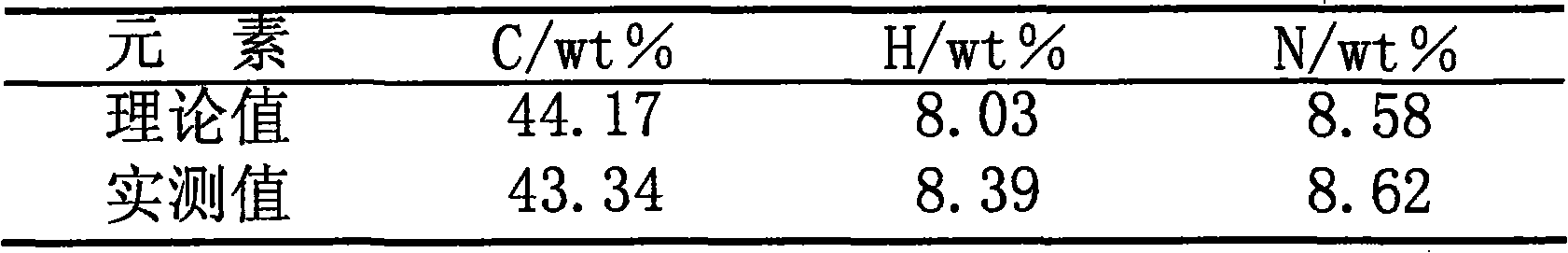Epoxy end group hyperbranched poly (amide-ester) and preparation method
A technology of hyperbranched polyester and hyperbranched polymer, which is applied in the field of epoxy-terminated hyperbranched polymer and its preparation method, can solve the problems of difficult acquisition of reaction raw materials, large-scale preparation and application impact, etc., achieve broad industrial promotion and application prospects, and be easy to use The effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
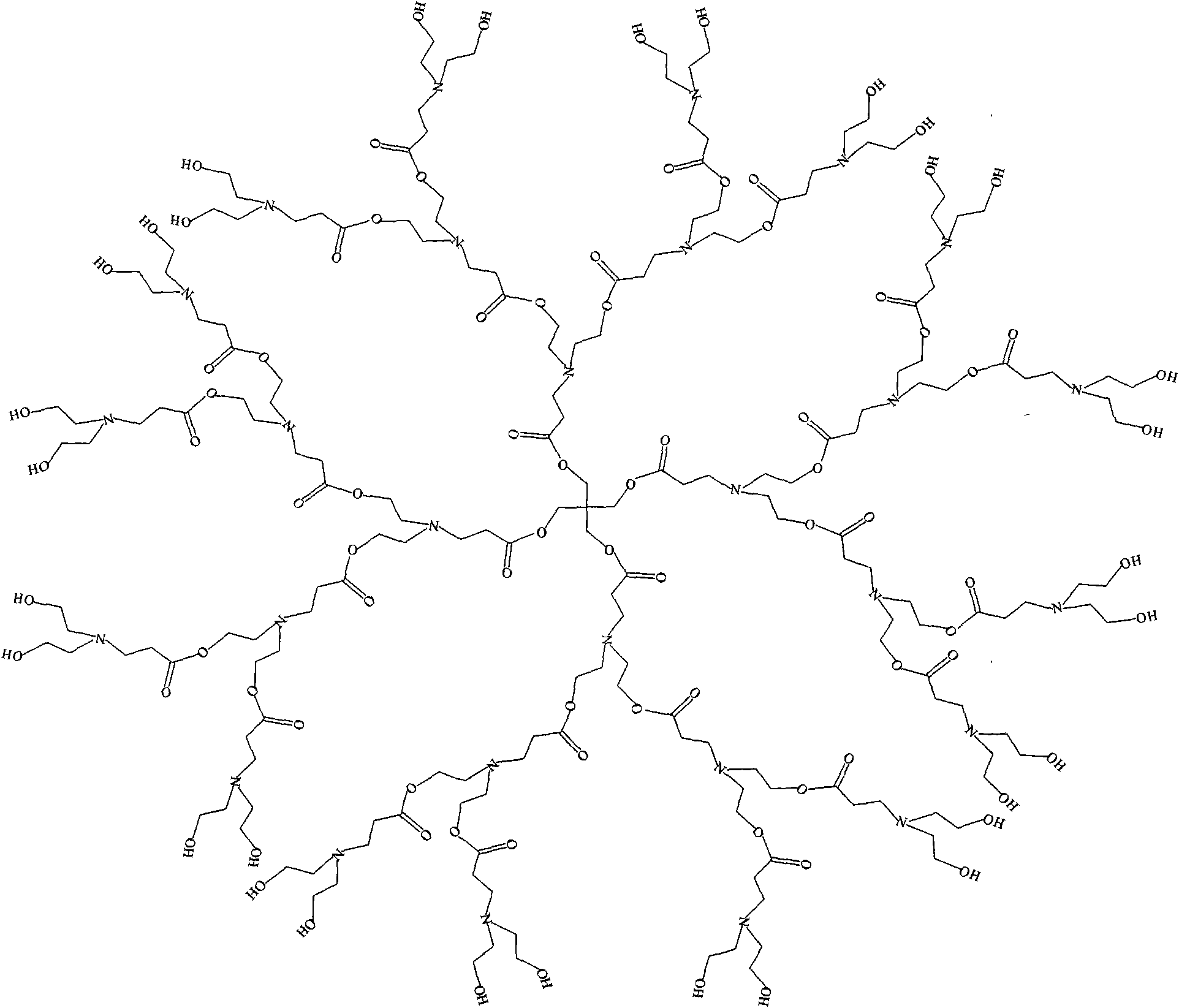
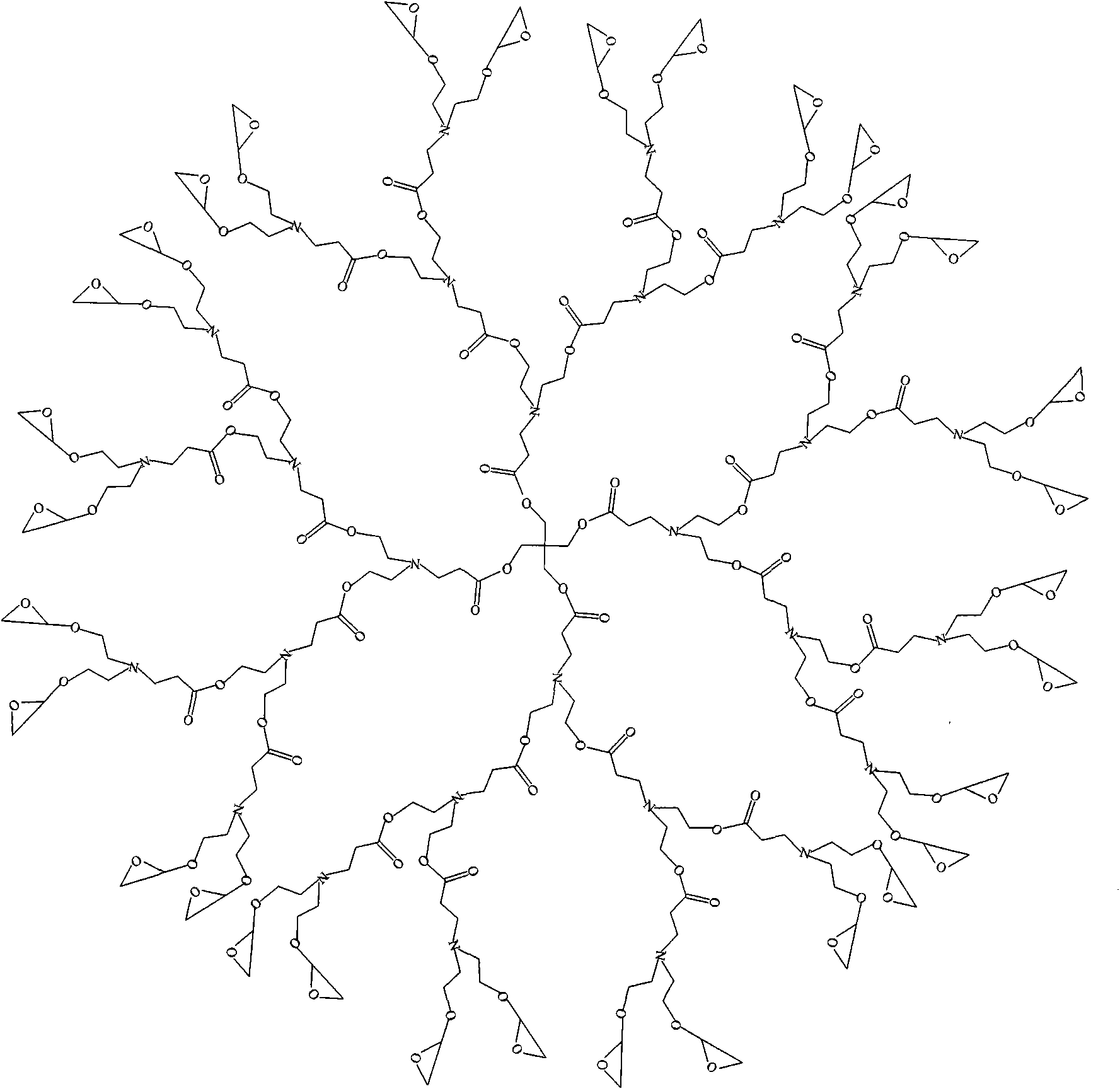
Image
Examples
Embodiment 1
[0034] Embodiment 1.N, the preparation of N-dihydroxyethyl-3-aminopropionic acid ethyl ester monomer
[0035]Using a 250ml three-necked flask reactor equipped with a stirrer and an oil bath and a nitrogen inlet, add 10.51g (0.10mol) diethanolamine and 5ml ethanol, then drop 10.01g in the three-necked flask with a dropping funnel at room temperature (0.10mol) ethyl acrylate, after the dropwise addition, the temperature was raised to 35°C, and the stirring reaction was continued for 4 hours, and then the ethanol was distilled under reduced pressure to obtain a colorless transparent oil, which was N,N-dihydroxyethyl-3- Ethyl aminopropionate monomer.
[0036] The synthesized monomer adopts gas chromatography spectrometer (Agilent 6890N) to analyze its content to be 94.97%; -1 、990cm -1 、910cm -1 The C=C bond peak in ethyl acrylate disappears at 939cm -1 The N-H characteristic peaks of diethanolamine disappeared at 1190.54cm -1 The absorption peak of C-N appears at 1731.93cm ...
Embodiment 2
[0037] Embodiment 2.N, the preparation of N-dimethylol-3-aminopropionic acid methyl ester monomer
[0038] Using a 250ml three-neck flask reactor equipped with a stirrer and an oil bath and a nitrogen inlet, add 8.6g (0.10mol) methyl acrylate, 7.7g (0.10mol) dimethanolamine and 5ml methanol, and the mixture is at room temperature and logical N , Stirring for 30 minutes, then raising the temperature to 35°C for 4 hours, and then vacuuming to remove methanol to obtain a colorless and transparent oil, which is N,N-dimethylol-3-aminopropionic acid methyl ester monomer.
[0039] The synthesized monomer was analyzed by infrared spectroscopy, and at 1445cm -1 、990cm -1 、910cm -1 The C=C bond peak in methyl acrylate disappears at 939cm -1 The N-H characteristic peaks of dimethanolamine all disappeared, and the results of measuring the content of monomer components by CHN-O-RAPID elemental analyzer of German Foss Heraeus company are shown in Table 1:
[0040] Table 1 Analysis resu...
Embodiment 3
[0043] Embodiment 3.N, the preparation of N-dihydroxyphenethyl-3-aminomethyl propionate isooctyl monomer
[0044] Using a 250ml three-neck flask reactor equipped with a stirrer and an oil bath and a nitrogen inlet, add 19.8g (0.10mol) of isooctyl methacrylate, 10.51g (0.10mol) of benzphenylethanolamine and 10ml of methanol, and the mixture is heated at room temperature And pass through N, under the condition of stirring for 30 minutes, then raise the temperature to 35 ° C for 4 hours, then vacuumize to remove methanol, and obtain a colorless and transparent oil, which is N, N-dihydroxyphenethyl-3-amino Isooctyl methylpropionate monomer.
[0045] The synthesized monomer was analyzed by infrared spectroscopy, and at 1445cm -1 The C=C bond peak in isooctyl methacrylate disappears at 939cm -1 The N-H characteristic peaks of dimethanolamine all disappeared, and the results of measuring the content of monomer components by CHN-O-RAPID elemental analyzer of German Foss Heraeus comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
| Theoretical hydroxyl value | aaaaa | aaaaa |
| Epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com