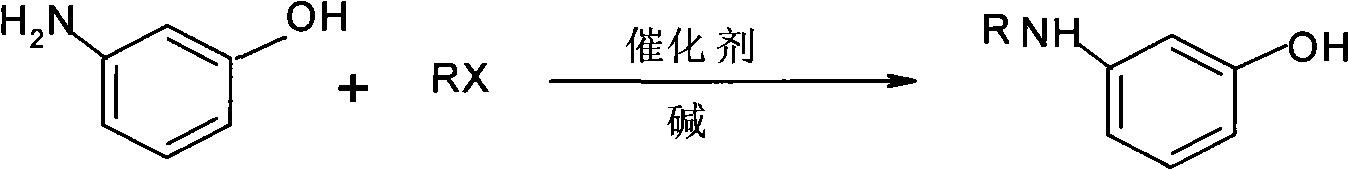
Preparation method of N-alkyl m-aminophenol
A technology of m-aminophenol and aminophenol, which is applied in the preparation of amino hydroxy compounds, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problem of low yield of N-alkyl m-aminophenol and primary conversion rate of resorcinol. Not high, not suitable for large-scale production and other problems, to achieve the effect of high selectivity, less impurities, easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
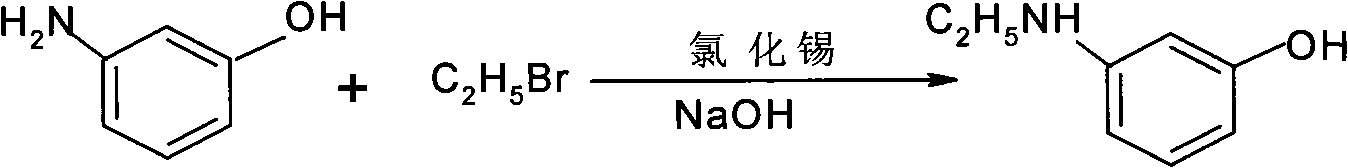
[0025] Embodiment 1: prepare a kind of N-alkyl m-aminophenol, be main raw material with m-aminophenol and the bromoethane of general formula RX, use water as solvent, make deacidifying agent with NaOH, tin chloride is made selectivity catalyst;
[0026] The chemical formula of the reaction is as follows:
[0027]
[0028] Among them, R is C 2 h 5 , X is Br;
[0029] Add 1.0ml m-aminophenol, 1.25ml bromoethane, 327g water and 1.09g tin chloride in a 1500ml four-necked bottle, the quality of 1.0ml m-aminophenol is 109g, the quality of 1.25ml bromoethane is 136.5g, Heat up to 50°C and start reflux, then slowly add 200g of 30% NaOH solution dropwise to the bottle, wherein the NaOH content is 1.5mol, after adding the NaOH solution, continue to react for 7 hours, and use gas chromatography to detect and track the reaction , when the content of m-aminophenol <0.5%, the reaction solution is obtained, add 240ml toluene to the bottle, carry out standing, sedimentation, extraction...
Embodiment 2
[0035] Embodiment 2: prepare a kind of N-alkyl m-aminophenol, take m-aminophenol and bromoisobutane whose general formula is RX as main raw materials, use isobutanol whose general formula is ROH as solvent, and use KOH as desorbent Acid agent, tin chloride as selective catalyst;
[0036] The chemical formula of the reaction is as follows:
[0037]
[0038] Wherein, R is isobutyl, X is Br;
[0039] Add 1.0ml m-aminophenol, 1.1ml bromoisobutane, 54.5g isobutanol and 7.63g tin chloride to a 1500ml four-necked bottle, the quality of 1.0ml m-aminophenol is 109g, 1.1ml bromoisobutane The quality of the product is 150.7g, and the temperature is raised to 70°C to start reflux, and then 128.3g of KOH solution with a concentration of 48% is slowly added dropwise to the bottle, wherein the content of KOH is 1.1mol. After adding the KOH solution, the reaction is continued for 5 hours. Detect and follow the reaction situation with gas chromatography, when m-aminophenol content<0.5%, o...
Embodiment 3
[0045] Embodiment 3: prepare a kind of N-alkyl m-aminophenol, be main raw material with m-aminophenol and general formula RX bromoisopentane, be the isoamyl alcohol of general formula ROH as solvent, use Na 2 CO 3 As deacidification agent, tin chloride as selective catalyst;
[0046] The chemical formula of the reaction is as follows:
[0047]
[0048] Wherein, R is isopentyl, X is Br;
[0049] Add 1.0ml of m-aminophenol, 1.0ml of bromoisopentane, 218g of isoamyl alcohol and 5.45g of tin chloride in a 1500ml four-necked bottle. Quality is 151g, is warming up to 90 ℃ and begins to reflux, then slowly adds 424g concentration to the bottle and is 20% Na 2 CO 3 solution, where Na 2 CO 3 The content is 0.8mol, after adding Na 2 CO 3 After the solution, continue to react for 2 hours, use gas chromatography to detect and track the reaction situation, when the m-aminophenol content < 0.5%, the reaction solution is obtained, add 300ml toluene to the bottle, let stand, settle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com