Method for synthesizing iron phosphate with doped metallic elements
A technology of metal elements and synthesis methods, applied in the direction of electrical components, electrode manufacturing, battery electrodes, etc., can solve problems such as high price, limited wide-scale use, and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
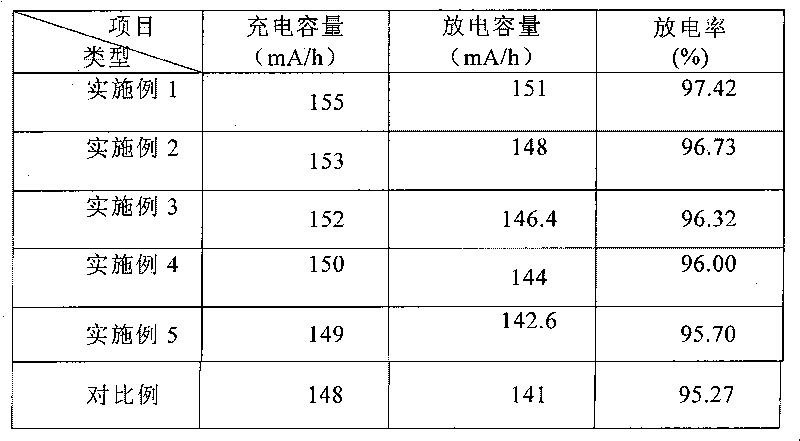
Embodiment 1
[0006] (1) With ferric nitrate as the iron source and nickel sulfate as the nickel source, the molar ratio of n(Fe):n(M)=0.8:0.2 is used to accurately weigh each material, and an appropriate amount of distilled water is added to prepare iron and a mixed aqueous solution of nickel; (2) prepared into a 2.5mol / L solution. With 1 mol / L of ammonium dihydrogen phosphate and 1.5 mol / L of ammonium phosphate as the phosphorus source; (3) using 10 mol / L of ammonia as a complexing agent and adjusting the pH value of the reaction system; (4) the above steps (1) The total molar concentration of the mixed aqueous solution of middle iron is 1.0mol / L; (5) using a volume of 2L reactor, the reaction control reaction temperature is 95 ℃, and the iron mixed solution containing doped Ni element is respectively mixed with a precision electronic metering pump The flow rate of 10ml / min, the phosphorus solution is pumped into the reactor at the flow rate of 4.2ml / min, the flow rate of the ammonia solu...
Embodiment 2
[0008] (1) Ferrous sulfate of iron is used as iron source, and vanadium chloride is used as vanadium source. According to the ratio of molar ratio n(Fe):n(M)=0.98:0.02, accurately weigh each material, add appropriate amount of distilled water, and prepare a mixed aqueous solution of iron and vanadium elements; (2) prepare a 1mol / L solution . With 0.5mol / L of ammonium phosphate and 0.5mol / L of trisodium phosphate as the phosphorus source; (3) using 6mol / L of ammonia as a complexing agent and adjusting the pH value of the reaction system; (4) the above steps (1) The total molar concentration of the mixed aqueous solution of iron is 0.5mol / L; (5) using a volume of 2L reactor, the reaction control reaction temperature is 40 ° C, and the iron mixed solution containing doped V element is respectively mixed with a precision electronic metering pump. The flow rate of 20ml / min, the phosphorus solution is pumped into the reactor at the flow rate of 12ml / min, the flow rate of the ammoni...
Embodiment 3
[0010] (1) Ferric nitrate is used as the iron source, and cobalt sulfate is used as the cobalt source. According to the ratio of molar ratio n(Fe):n(M)=0.95:0.05, accurately weigh each material, add an appropriate amount of distilled water, and prepare a mixed aqueous solution of iron and cobalt elements; (2) prepare a 2mol / L solution . With ammonium hydrogen phosphate 1.0mol / L, trisodium phosphate 1.0mol / L mixed solution is phosphorus source; (3) with the ammoniacal liquor of 6mol / L as complexing agent and adjust the pH value of reaction system; (4) above step (1 ) The total molar concentration of the mixed aqueous solution of iron in ) is 0.5mol / L; (5) using a volume of 2L reactor, the reaction temperature is controlled to be 65 ℃, and the iron mixed solution containing doped Co element is separately mixed with a precision electronic metering pump With the flow rate of 20ml / min, the phosphorus solution is pumped into the reactor at the flow rate of 12ml / min, the flow rate o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com