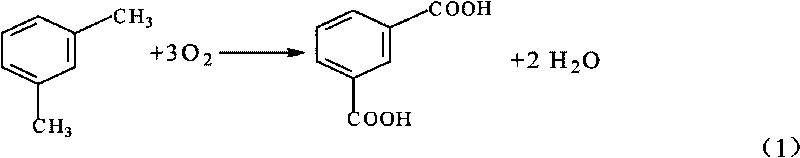
Process for producing isophthalic acid
A technology of isophthalic acid and m-xylene, which is applied in the preparation of carboxylate, the preparation of organic compounds, organic compounds/hydrides/coordination complex catalysts, etc. The problems of rising production costs, etc., can reduce the combustion reaction rate, minimize environmental pollution, and reduce equipment corrosion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The coconut shell gac of 15g is soaked in the phosphomolybdovanadic acid (H 6 PMo 9 V 3 o 40 ·5H 2 O) in an aqueous solution, heated to reflux for 6 hours, filtered, washed with hot water until neutral, drained, dried at 110°C for 4 hours, then activated at 350°C for 12 hours under the protection of nitrogen to obtain a loading capacity of 10% (weight) of H 6 PMo 9 V 3 o 40 / C Catalyst.
[0034] The experiment was carried out in a stirred reactor with a volume of 1000ml, adding 400ml of glacial acetic acid, 50g of water, 25g of m-xylene, 5g of the previously prepared 40-60 mesh activated carbon supported heteropolyacid catalyst, the reaction temperature was 210°C, and the pressure was 13MPa. The flow rate of air is 1 L / min, the reaction is 30 minutes, the conversion rate of m-xylene is 100%, and the yield of isophthalic acid reaches 95%.
Embodiment 2
[0036] The preparation method of activated carbon-supported heteropolyacid catalyst is as in Example 1.
[0037] The experiment was carried out in a fixed-bed reactor with a height of 80 cm and an inner diameter of 2 cm, which contained 10-20 meshes of activated carbon-loaded heteropolyacid catalysts. The gas-liquid two-phase flowed through the reactor from bottom to top, and the flow rate of the liquid was 10ml / min. The flow rate of air 500ml / min, in the liquid feed, m-xylene 15% (weight), water 20% (weight), all the other are acetic acid, 210 ℃ of reaction temperature, pressure 13MPa, m-xylene conversion rate 50%, m-xylene Diformic acid yield reached 49%.
Embodiment 3
[0039] 15g of coal-based activated carbon was immersed in 20% phosphotungstic acid (H 3 PW 12 o 40 ) in an aqueous solution, heated to reflux for 4 hours, filtered, washed with hot water until neutral, drained, dried at 110°C for 5 hours, and activated at 300°C for 10 hours under the protection of nitrogen to obtain a loading capacity of 15 % (weight) of H 3 PW 12 o 40 / C Catalyst.
[0040] Experimental research was carried out under the same conditions as in Example 1. After 35 minutes of reaction, the conversion rate of m-xylene was 100%, and the yield of isophthalic acid reached 94%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com