Liquid crystal compound containing fluoropyrimidine and application thereof
A technology of liquid crystal compounds and pyrimidines, which is applied in the direction of liquid crystal materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of low driving voltage refractive index dielectric anisotropy, high viscosity, low threshold voltage, etc., and achieve large optical Anisotropy, good compatibility, and improved properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
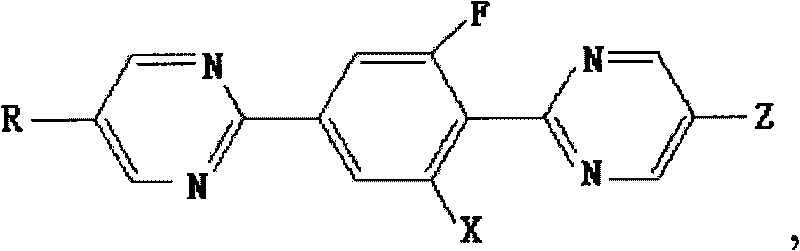
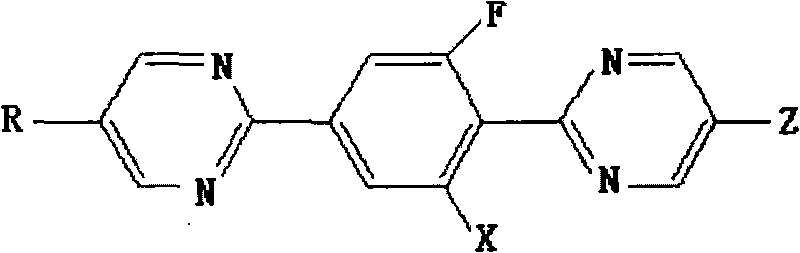
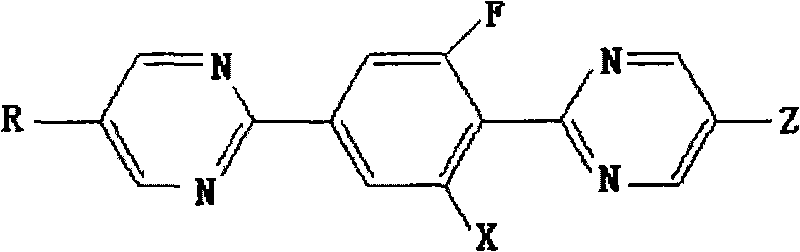
[0037] Example 1 Synthesis of 2-[4-(5-propylpyrimidine 2-)-2-fluorophenyl]-5-propylpyrimidine
[0038] When R and Z are respectively propyl and X are hydrogen, the compound represented by formula I is 2-[4-(5-propylpyrimidine 2-)-2-fluorophenyl]-5-propyl-pyrimidine, Its synthesis process is carried out according to the following sequence of steps:
[0039] a1. Compound 1-2: Synthesis of valerenamine
[0040] Add 200g of potassium carbonate and 180ml of morpholine into a 500ml three-necked bottle, install a stirrer, a thermometer, and a constant pressure dropping funnel, cool the outside of the bottle to below 5°C, and start adding 86g of n-butyraldehyde (compound 1-1) dropwise for about 30 minutes The dropwise addition is completed; keep at 0-10°C for 8 hours, suction filter the reaction solution, rinse the funnel several times with 1000ml petroleum ether (90-105°C) to concentrate the filtrate, and collect the colorless liquid at 76-86°C / 20mmHg by distillation under reduced pre...
Embodiment 2
[0057] Example 2 Synthesis of 2-[4-(5-propylpyrimidine 2-)-2-fluorophenyl]-5-propylpyrimidine
[0058] In the formula II, R is a propyl group, and when X is a hydrogen group, the compound is 2-[4-(5-propylpyrimidine 2-)-2-fluorophenyl]-5-propyl-pyrimidine, and its synthetic method is as follows The steps are performed in sequence:
[0059] a 2. Compound 2-2: Synthesis of 1-dimethylamino-3-dimethylimino-2-fluoropropene perchlorate
[0060] In a 250ml three-neck flask equipped with a stirring and reflux condenser, 20g of fluoroacetic acid (compound 2-1) and 100g of thionyl chloride were added; the reaction was refluxed for 6h under heating. Evaporate excess solvent to obtain fluoroacetyl chloride (standby); add 200ml N, N-dimethylformamide in another 500ml three-necked flask equipped with stirring and reflux condenser, and add phosphorus oxychloride 60ml and After the addition of the spare fluoroacetyl chloride, react at 20-30°C for 5 hours, pour the reaction solution into 700...
Embodiment 3
[0069] Example 3 Synthesis of 2-[4-(5-propylpyrimidine-2-)-2-fluorophenyl]-5-trifluoromethyl-pyrimidine
[0070] When R is a propyl group and X is a hydrogen group, the compound shown in formula III is 2-[4-(5-propylpyrimidine 2-)-2-fluorophenyl]-5-trifluoromethyl-pyrimidine, and its synthesis The method proceeds in the following order of steps:
[0071] a3. Compound 3-1: Synthesis of 2-[4-(5-bromopyrimidine-2-)-3-fluorophenyl]-5-propyl-pyrimidine
[0072] Add 2-fluoro-4-(5-propylpyrimidin-2-yl) benzamidine hydrochloride (compound 1-6) 29.5g successively in a 500ml three-necked flask equipped with mechanical stirring, reflux condenser and thermometer , bromomalondialdehyde 15g, methanol 200mL and sodium methoxide 8g, heat to reflux for 8h, cool, filter, then use 150ml toluene, 300ml petroleum ether hot melt product overheated silica gel chromatography column, use 80ml toluene and 160ml petroleum ether mixed solution Wash the column, combine the organic phases and concentrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com