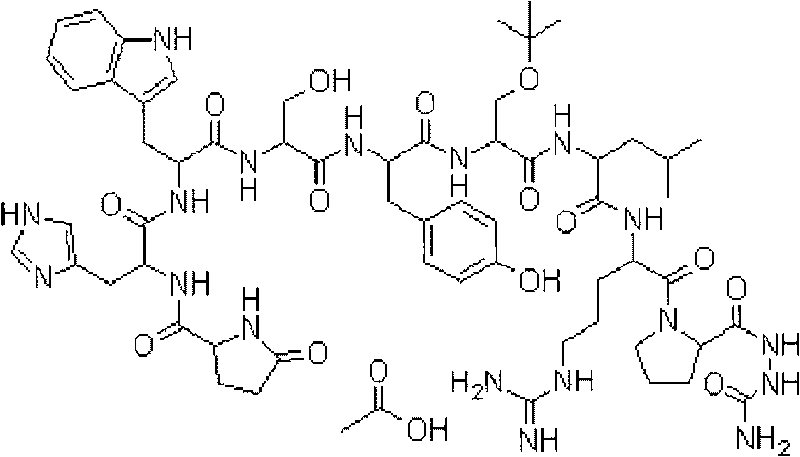
Goserelin release microsphere preparation, preparation method and detecting method thereof
A slow-release microsphere preparation and microsphere technology, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve problems that have not been reported, and achieve convenient clinical use, The effect of improving compliance and reducing the number of injections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The preparation method of microsphere preparation can be realized by methods such as phase separation method, drying method in water, spray drying method, wherein the drying method in water is preferred, and its basic process: dissolve lactide-glycolide copolymer in dichloromethane or acetic acid An organic solvent such as ethyl ester is used to obtain an organic phase, and goserelin is dissolved in a gelatin solution to form a water phase. Mix well to form colostrum, prepare an aqueous solution of a stable emulsifier, disperse the W / O colostrum in the solution containing a stable emulsifier, stir to form a W / O / W double emulsion, evaporate the film, stir and centrifuge for washing, and add manna Alcohol is used as a skeleton agent and freeze-dried to obtain goserelin microspheres containing double-layer organic phases.
[0054] The preparation method of the goserelin sustained-release microsphere preparation of the present invention is characterized in that two polymer ...
Embodiment 1
[0058] Weigh 750mg lactide-glycolide copolymer (75:25, molecular weight 15000) and dissolve in 5ml dichloromethane as organic phase. 1 , Weigh 125mg goserelin and dissolve in 2.5ml 20% gelatin aqueous solution as the water phase W 1 , the organic phase and the aqueous phase are dispersed at a high speed at 40°C to become W 1 / O colostrum, then stir and disperse the colostrum in 250mg / 2ml polylactic acid (molecular weight 25000) dichloromethane solution, slowly add the above solution dropwise to 500ml containing 0.1% polyvinyl alcohol 205, stir and disperse at a temperature of about 40°C 1h, lower the temperature and stir for 6h, volatilize to remove the organic solvent, filter and wash with water, add 20% mannitol in total as the skeleton agent to freeze-dry, and pack into goserelin microsphere preparations with an actual drug loading of 36mg, particle size 50 -100um, sterilization method, using 60 Sterilized by Co gamma radiation. One dose is injected subcutaneously once a...
Embodiment 2
[0061] Dissolve 1000 mg of lactide-glycolide copolymer (50:50, molecular weight 20000) in 5 ml of dichloromethane as organic phase O 1 , Weigh 150mg goserelin and dissolve in 2.5ml 20% gelatin aqueous solution as the water phase W 1 , heated to 50°C, and the organic phase and the aqueous phase were dispersed ultrasonically (35HZ) at 50°C for 5min to become W 1 / O colostrum, then the colostrum is stirred and dispersed in the dichloromethane solution of the polylactic acid (molecular weight 25000) of 300mg / 2ml, the above solution is slowly added dropwise to 20ml containing 0.5% polyvinyl alcohol 205, and the homogenizer is dispersed at a high speed (10000rpm / min), evaporating on a rotary evaporator at 0°C for 30min, transferring to an open instrument and stirring at room temperature for 12h, volatilizing to remove the organic solvent, filtering and washing with water, adding 20% mannitol as a skeleton agent to freeze-dry, and subpackaging into actual drug loading Goserelin m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com